Conference 22, Case 2
Signalment:
<30-day-old (neonate), female, Texel lamb, Ovis aries
History:
Death of 20, <30-day-old lambs was reported in a flock of 450 nonvaccinated Texel sheep in a farm in the department of Rivera, Uruguay, in October 2023 (spring season in the Southern Hemisphere). The flock was grazing natural grassland on a low terrain that used to be used for the cultivation of rice, and
following a severe flooding event, the affected lambs exhibited severe apathy and hemoglobinuria, progressing to death within 24-48 hours.
Gross Pathology:
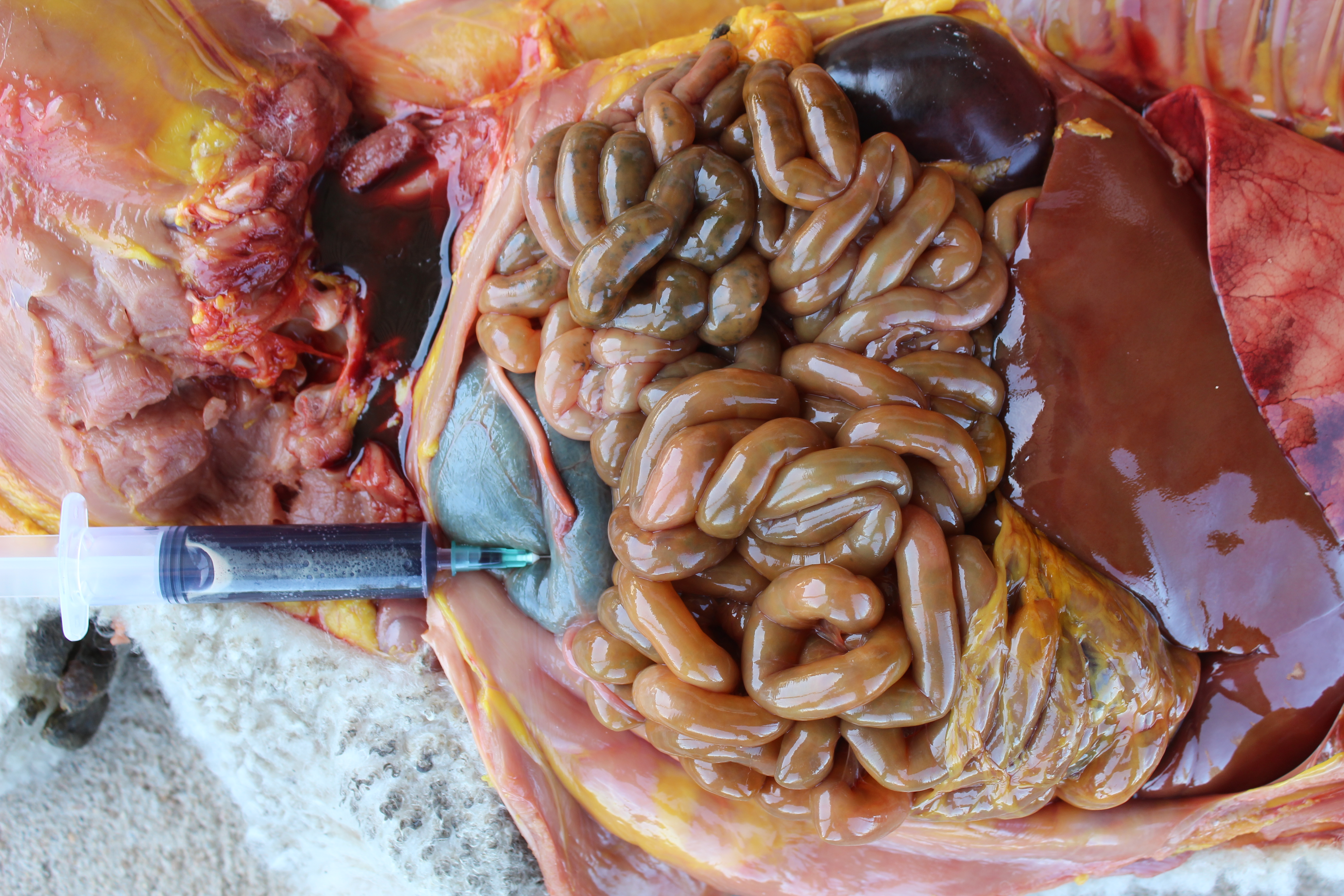
The main gross findings included yellowish discoloration of the subcutaneous and adipose tissues (jaundice), diffuse bilateral dark-red discoloration of the kidneys with dark-red urine filling the urinary bladder (hemoglobinuria), pale skeletal muscles (anemia) (Figure 1), dark pink to red mottled lungs with rubbery/meaty texture and intraluminal bronchial and tracheal froth (pulmonary edema).
Laboratory Results:
|
Tissues |
||
|
Tests for detecting Leptospira |
Liver |
Kidney |
|
Immunohistochemistry* |
Positive |
Negative |
|
qPCR for pathogenic Leptospira spp. (targeting the lipL32 gene) |
Positive |
Positive |
|
PCR targeting the 16S rDNA gene followed by sequencing for species identification |
L. kirschneri |
L. kirschneri |
*Using Leptospira multivalent fluorescent antibody conjugate (LEP-FAC) as primary antibody.
Microscopic Description:
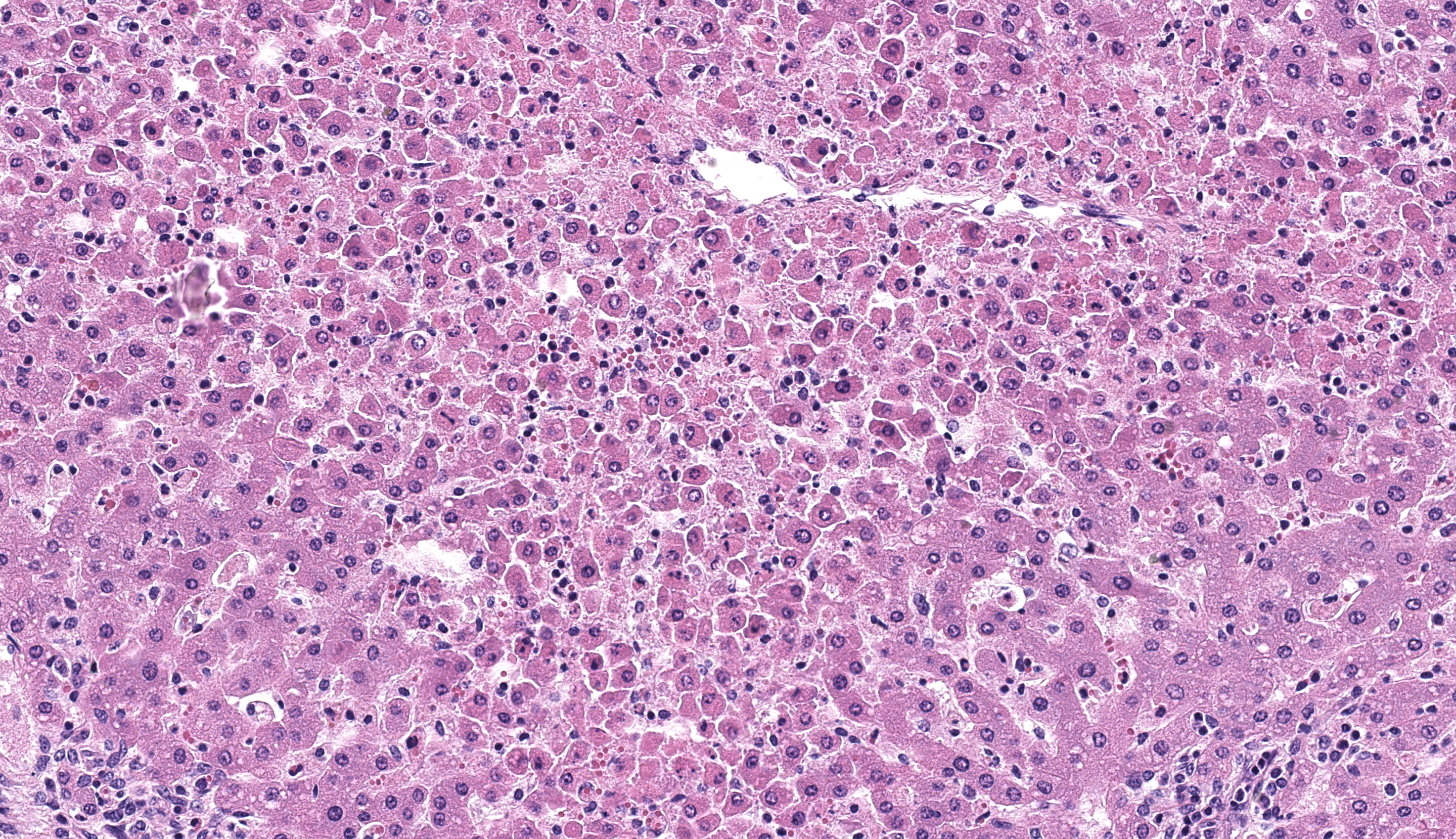
Liver: diffusely there is disruption of the histoarchitecture of hepatic cords in the centrilobular (periacinar) areas with a bridging pattern. Within these regions hepatocytes are frequently dissociated and individualized from the hepatic cords and are either swollen with vesicular nucleus (hydropic degeneration) or shrunken with angular cell borders and hypereosinophilic cytoplasm and nuclear pyknosis or karyorrhexis (necrosis) (Figure 2). In these areas there is multifocal infiltration of neutrophils, lymphocytes and histiocytes, occasionally grouping around necrotic hepatocytes, although similar inflammatory infiltrates are present in the sinusoids of the midzonal and periportal regions where hepatocytes are preserved. Portal tracts are moderately and multifocally expanded by inflammatory cells, notably histiocytes, lymphocytes and rare neutrophils, and/or fibroblasts embedded in a loose extracellular collagenous matrix (fibrosis/fibroplasia).
Contributor’s Morphologic Diagnosis:
- Liver: multifocal random neutrophilic and lymphohistiocytic hepatitis with severe diffuse acute periacinar hepatocellular necrosis, lamb.
- Liver: hepatitis, portal, lymphocytic and histiocytic, with fibrosis, lamb
Contributor’s Comment:
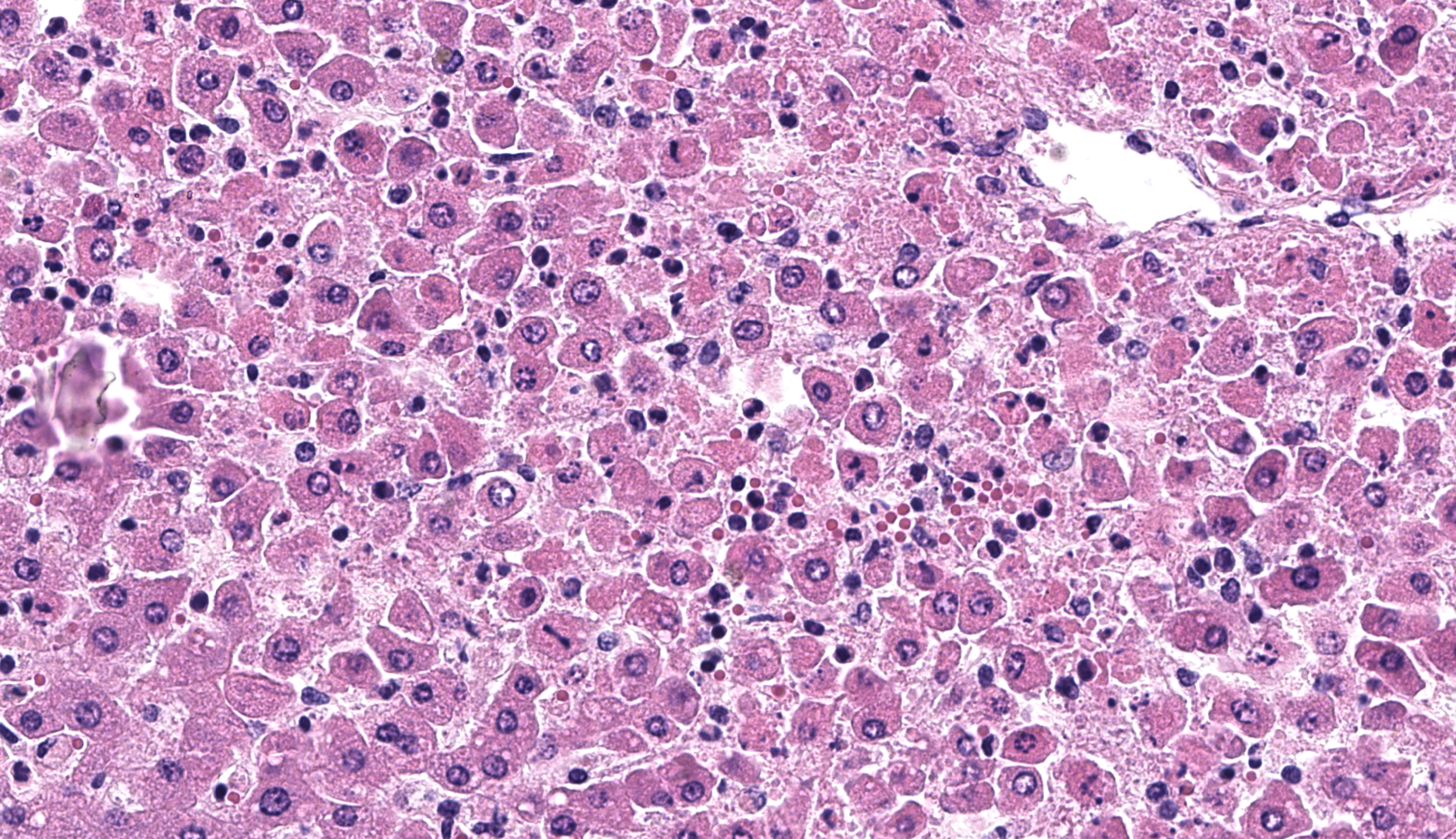
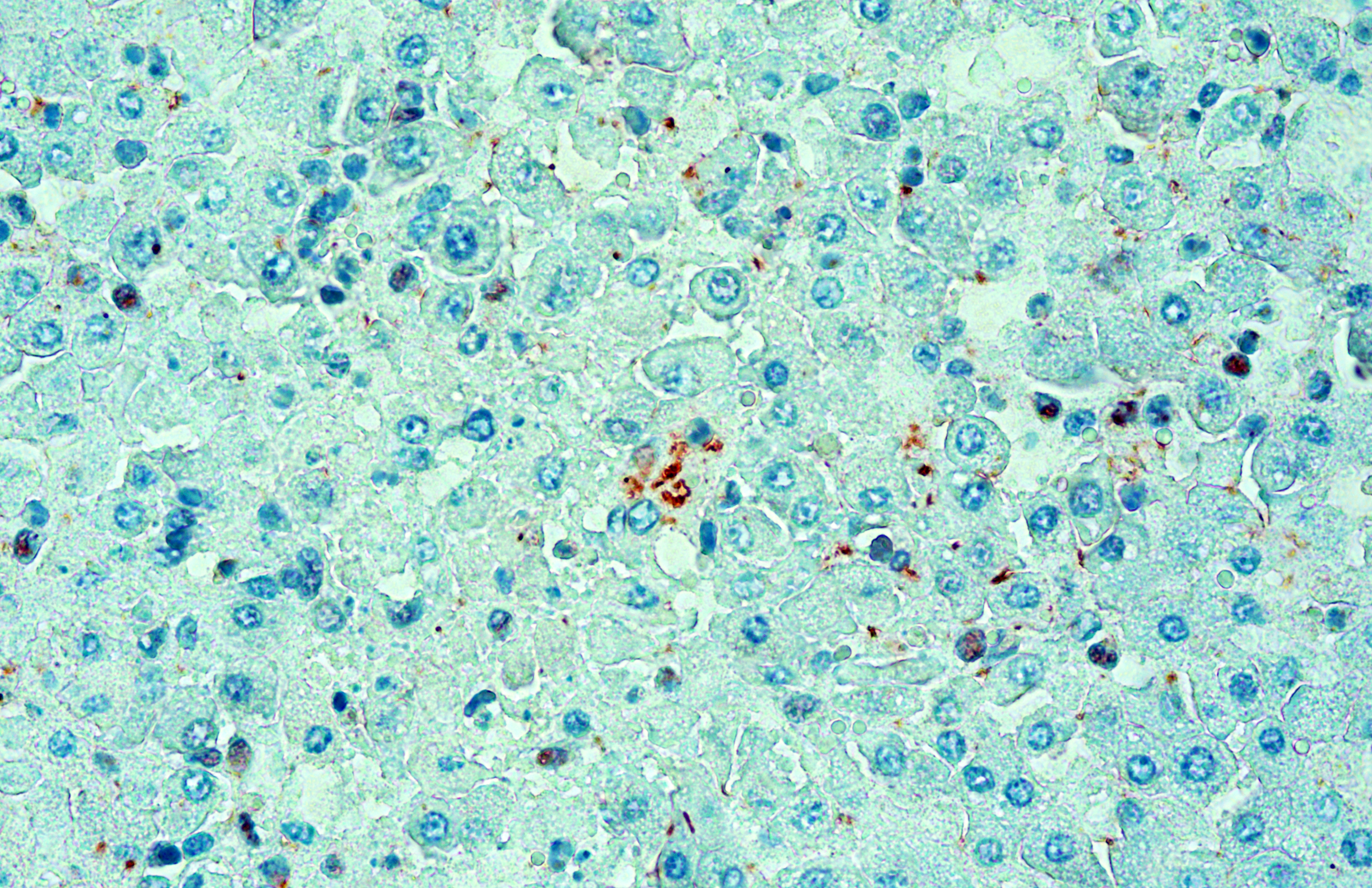
The clinical signs and gross pathological findings in this lamb were highly suggestive of acute (likely intravascular) hemolysis. Differential diagnoses include “yellow lamb disease” (a poorly characterized enterotoxemia presumably caused by Clostridium perfringens alpha toxin), Mycoplasma ovis infection, and toxic plants causing hemolysis (i.e. Allium spp., Brassica spp., Indigofera spp., Urochloa arrecta).2,3,8,9 In older sheep, chronic copper toxicity is characterized by similar clinical and pathological findings.2 In this case, an etiologic diagnosis of leptospirosis was established based on intralesional identification of Leptospira spp. antigen by immunohistochemistry in the liver (Figure 3), and molecular identification of Leptospira kirschneri in the liver and kidney (see laboratory results section).
The nature and distribution of the microscopic lesions in the liver of the lamb, notably diffuse acute periacinar hepatocellular degeneration/necrosis, suggest that they probably resulted, at least in part, from hypoxia perhaps secondary to hemolytic anemia. However, a direct action of leptospiral invasion and multiplication in the liver, as highlighted immunohistochemically, probably contributed to the inflammatory and necrotizing lesions seen in the hepatic parenchyma. It was not clear whether the portal lesions in this case were due to Leptospira infection or a pre-existing incidental finding. No Leptospira antigen could be clearly identified in the portal tracts or bile ducts. Notably, despite the jaundice seen grossly, no significant bile stasis was observed histologically in the liver, suggesting pre-hepatic jaundice probably resulting from hemolysis.
Leptospirosis is a zoonotic disease of worldwide distribution caused by spirochetes in the genus Leptospira. The taxonomy of the genus has evolved enormously in recent years, and now contains several pathogenic species such as L. interrogans, L. borgpetersenii, L. noguchii, and L. kirschneri, within which more than 350 serovars have been identified.4 While a wide range of wild and domestic animal species can be infected by a wide range of serovars, some serovars are adapted to a given animal species (maintenance hosts) and cause disease in another species (incidental hosts).1 The major host-adapted serovars are Hardjo in cattle and sheep, Icterohaemorrhagiae and Copenhageni in rats, Ballum in mice, Canicola in dogs, and Pomona and Bratislava in pigs. Dogs, cattle, pigs, and horses are the main incidental domestic animal hosts and suffer from the disease in diverse degrees from asymptomatic to lethal.4
The natural niche of pathogenic Leptospira spp. are the proximal renal tubules and the genital tract in certain maintenance hosts. Transmission might be direct through contact with urine, lochia, milk, genital mucosa (venereal transmission), or transplacental. Infection of incidental hosts is usually indirect, via environmental contamination by urine of carrier animals. Under ideal wet, warm, and neutral to slightly alkaline conditions leptospires may survive for weeks or months in water-logged soil or water. Thus, in temperate climates leptospirosis occurs especially in the wet season and the risk of exposure and infection is increased by heavy rainfall, agricultural irrigation and/or flooding. In tropical and subtropical areas, the disease can occur year-round.1,4
In incidental hosts, infection may cause severe acute or subacute systemic disease during bacteremia (leptospiremic phase), particularly in young animals. After leptospiremia has ceased, chronic disease can manifest as abortion, stillbirth, infertility, or recurrent uveitis. The acute/subacute systemic disease is clinically characterized by fever, jaundice, hemolytic anemia, hemoglobinuria, pulmonary congestion, and occasional meningitis.1
Leptospira genomes encode many proteins of unknown or poorly defined function and the molecular bases for virulence are poorly understood. Adhesion to host cells and components of the extracellular matrix likely plays a role in virulence. Flagellar motility, notably controlled by chemotaxis, is key to Leptospira virulence, as are several mechanisms allowing the bacteria to escape or resist the host immune response. How the lipopolysaccharide (LPS) contributes to virulence is still poorly known, but mutations that alter the LPS structure attenuate virulence. Pathogenic Leptospira spp. also produce sphingomyelinase-like enzymes with phospholipase C, hemolytic, and apoptotic activities. As these are absent in the saprophytic Leptospira spp. they are thought to contribute to virulence.4
Leptospirosis is a complex disease; its pathogenesis has not been fully elucidated and varies for different serovars in different animal species.1 Leptospires entry to the body through mucous membranes (conjunctival, oral, genital) or via damaged or compromised skin, without inducing notable lesions at the point of entry.4 The mechanisms used to enter the bloodstream through endothelial cells are not well understood; however, motility seems to be essential for pathogenic Leptospira spp. to cross tissue barriers, which is thought to occur through intracellular translocation. During the leptospiremic phase (hematogenous dissemination), which can last up to 7 days, the main symptom is fever, while clinicopathological findings frequently include leukocytosis and thrombocytopenia. Hemorrhages in the liver, kidneys, and/or lungs can develop in the acute phase of the disease, at which stage inflammation is usually absent.
After rapid hematogenous spread, leptospires interact through adhesins with a range of host tissue proteins in different organs.4 The pathogen multiplies especially well in the liver, kidney, lungs, placenta, mammary gland, and cerebrospinal fluid (CSF).1 Leptospira have developed strategies to evade host defenses and resist the complement system. Although generally considered an extracellular pathogen, it is also able to penetrate host cells and survive inside macrophages and other phagocytes involved in the innate immune response. Leptospires have also developed strategies to escape recognition by pattern-recognition receptors of the innate immune system that recognize microbe-associated molecular patterns (i.e. Nod-like and Toll-like receptors). However, the innate immune system can detect Leptospira and activate an immune response and the expression of cytokines.4 After tissue colonization, hepatitis, and hepatocellular death (apoptosis/necrosis) can be seen early in the disease associated with leptospiral invasion. In the kidneys, interstitial nephritis is seen, but can be quite discrete, whereas hemoglobinuria can occur in ruminants.4 Development of agglutinating and opsonizing antibodies after approximately 6-7 days clears the agent from most organs except from immunoprivileged sites such as the proximal tubules of the kidneys, the CSF, and the vitreous humor of the eyes. Certain serovars can also survive and establish chronic infection in the genital tract of maintenance hosts.1
In sheep, Leptospira infection is usually asymptomatic, although severe disease occurs sporadically in young animals. In neonate lambs, leptospirosis is usually characterized by leptospiremia with hemolysis, anemia, and death. There is little information on the species and serovars involved in fatal cases of leptospirosis in sheep. In Uruguay, sporadic outbreaks of acute fatal leptospirosis in lambs associated have been associated with L. interrogans serogroup Pomona serovar Kennewicki.5 L. kirschneri, the species detected in the case described herein, has been identified as one of the dominant Leptospira species (along with L. interrogans) associated with human leptospirosis in central Malaysia.7
Regardless of the acting species, it is important to determine the serovars involved in outbreaks of leptospirosis and/or infecting animals and humans in different geographic regions, so that circulating serovars can be included in vaccines, considering that immunity is serovar specific. Unfortunately, the serovar involved in the ovine case presented here could not be identified as attempts to culture and isolate this labile and fastidious organism were unsuccessful. Of note, the serovar Kennewicki which has been isolated from sheep,5 cattle10 and humans6 in Uruguay is not included in the locally available commercial vaccines for livestock.
Contributing Institution:
Instituto Nacional de Investigación Agropecuaria (INIA), Route 50, Kilometer 11, La Estanzuela, Colonia 70006, Uruguay.
www.inia.uy
JPC Diagnosis:
Liver: Hepatitis, necrotizing, acute, diffuse and centrilobular, moderate, with hepatocellular disassociation.
JPC Comment:
The contributor provides an excellent summary of leptospirosis that weaves in some of the salient features of this case. Histologic changes were apparent even from low-magnification and we agree with the contributor that the underlying pathogenesis is acute centrilobular hepatitis that is probably augmented by hypoxic effects. Although anemia is more of a clinical diagnosis than a histologic one, we could not help but notice the paucity of erythrocytes within sinusoids that corresponds to the intravascular hemolysis characteristic of this agent. Dissociation (individualization) of hepatocytes was another important feature of this case which has been attributed to hepatic infectious with several species of Leptospira.11 These bacteria can infiltrate the space of Disse and cause dissociation of hepatocytes through physical disruption and breakdown of intracellular tight junctions. A second consequence of the loss of tight conjunctions is leakage of bile from the canaliculus – this is a form of intrahepatic cholestasis that lacks dilatation of bile canaliculi.11 In this case, the lack of bile plugs despite systemic icterus fits with this interpretation.
We also ran a Masson’s trichrome stain to evaluate liver structure and concurrent pathology. We differed from the contributor in that we did not observe hepatic fibrosis, to include within centrilobular regions with hepatocyte loss. This argues against a prolonged event, to include secondary hypoxic changes and/or sinusoidal outflow obstruction (see Conference 12, Case 2 of this year for a relevant example). Finally, we debated the relevance of periportal cellular infiltrates. While some participants considered oval cell hyperplasia, the group consensus was for extramedullary hematopoiesis given the age of this animal and concurrent loss of erythrocytes necessitating demand.
The lack of histologic renal changes in this young lamb likely reflected a very acute course of disease and an insufficient time course for bacteremia to bring the agent to the lumina of renal tubules via the renal vasculature. That stated, the positive qPCR result in the kidney is intriguing, and we wonder whether the detection of nucleic acid therein could reflect cross-contamination.
References:
- Cianciolo RE, Mohr CF. Urinary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th Vol 2. Amsterdam, The Netherlands: Elsevier; 2016:433–439.
- Cullen JM, Stalker MJ. Liver and Biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 2. Amsterdam, The Netherlands: Elsevier; 2016:258–352.
- Giannitti F, Macias Rioseco M, García JP, et al. Diagnostic exercise: hemolysis and sudden death in lambs. Vet Pathol. 2014;51(3):624–627.
- Goarant C, Adler B, de la Peña Moctezuma A. Leptospira. In: Prescott JF, MacInnes JI, Van Immerseel FV, Boyce JD, Rycroft AN, Vázquez-Boland JA, eds. Pathogenesis of Bacterial Infections in Animals. 5th Hoboken, NJ, USA: Wiley Blackwell; 2023: 502–527.
- Hamond C, Silveira CS, Buroni F, et al. Leptospira interrogans serogroup Pomona serovar Kennewicki infection in two sheep flocks with acute leptospirosis in Uruguay. Transbound Emerg Dis. 2019; 66(3):1186–1194.
- Meny P, Menéndez C, Quintero J, et al. Characterization of Leptospira isolates from humans and the environment in Uruguay. Rev Inst Med Trop Sao Paulo. 2017; 59:e79.
- Philip N, Affendy NB, Ramli SNA, et al. Leptospira interrogans and Leptospira kirschneri are the dominant Leptospira species causing human leptospirosis in Central Malaysia. PLoS Negl Trop Dis. 2020;14(3): e0008197.
- Uzal FA, Giannitti F, Asin J. Yellow lamb disease (Clostridium perfringens Type A enterotoxemia of sheep): A review. Animals (Basel). 2022; 12(12): 1590.
- Windsor PA. Anaemia in lambs caused by Mycoplasma ovis: Global and Australian perspectives. Animals (Basel). 2022; 12(11): 1372.
- Zarantonelli L, Suanes A, Meny P, et al. Isolation of pathogenic Leptospira strains from naturally infected cattle in Uruguay reveals high serovar diversity, and uncovers a relevant risk for human leptospirosis. PLoS Negl Trop Dis. 2018; 12(9):e0006694.
- Miyahara S, Saito M, Kanemaru T, Villanueva SY, Gloriani NG, Yoshida S. Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil's disease. Int J Exp Pathol. 2014 Aug;95(4):271-81.