Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 22
15 April, 2020
CASE IV: WSC 1920 Conf 22 Case 4 DVD (JPC 4100234).
Signalment: 26-year-old, male intact Geoffroy?s spider monkey (Ateles geoffroyi)
History: The previous year, this animal had episodes of depression and diarrhea and was diagnosed with Campylobacter hyointestinalis. At that time, treatment led to complete resolution of signs. The following year, the monkey presented with lethargy and loose stool. Weight loss of 2 kg had occurred over the previous two months and symptomatic treatment including fluids and antibiotics was provided. The animal died the following day.
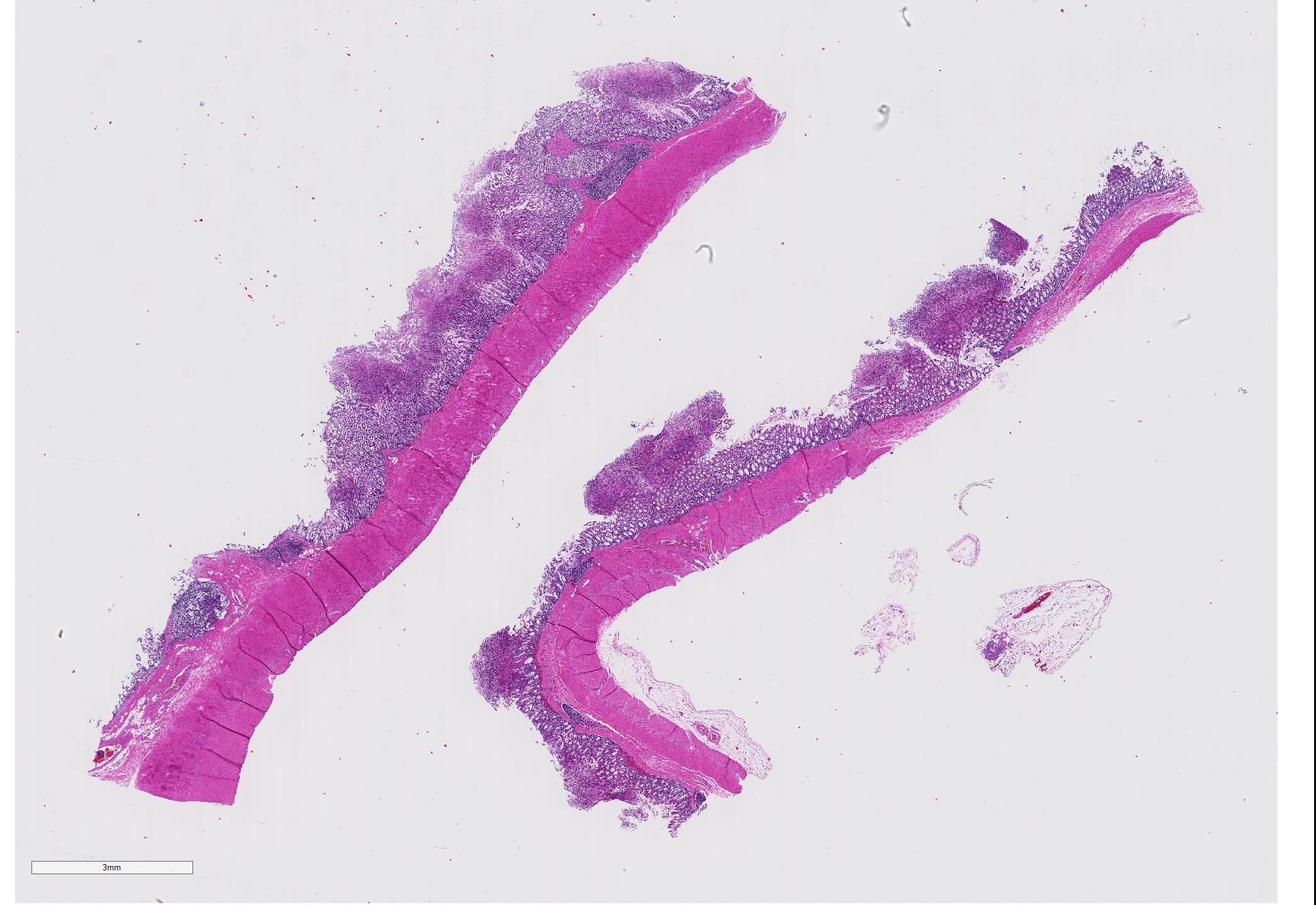
Gross Pathology: On necropsy, the markedly dilated large intestine contained a large amount of homogenous, brownish, turbid, thick fluid. The most aborad ileal mucosa, the cecal mucosa, and large portions of the colonic mucosa were covered by pale brown membranous material that was easily removable. Underneath this material, the mucosa was reddened but appeared otherwise intact. While the cecal mucosa was almost entirely coated by the pseudomembraneous material, particularly in the colon, the pseudomembraneous material was distributed multifocally in numerous geographical to round, up to 3 to 4mm in diameter convex deposits that were roughly evenly spaced.
Laboratory results:
Bacteriology ? aerobic and anaerobic cultures were negative. A culture for Clostridium difficile specifically was positive. There was no evidence of a parasitic infection based on the negative result of fecal flotation. Viral particles were not detected in the colonic content at electron microscopy.
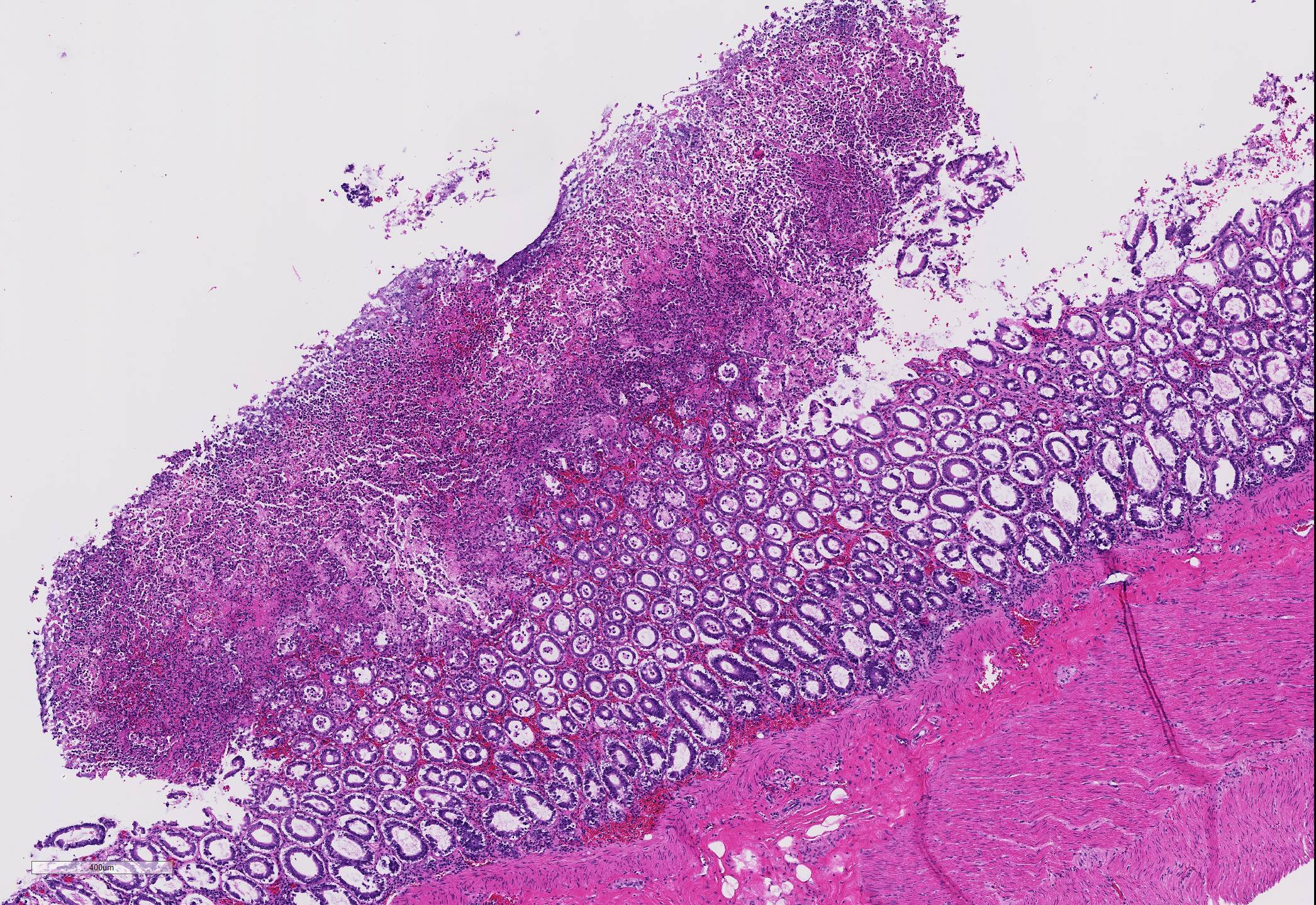
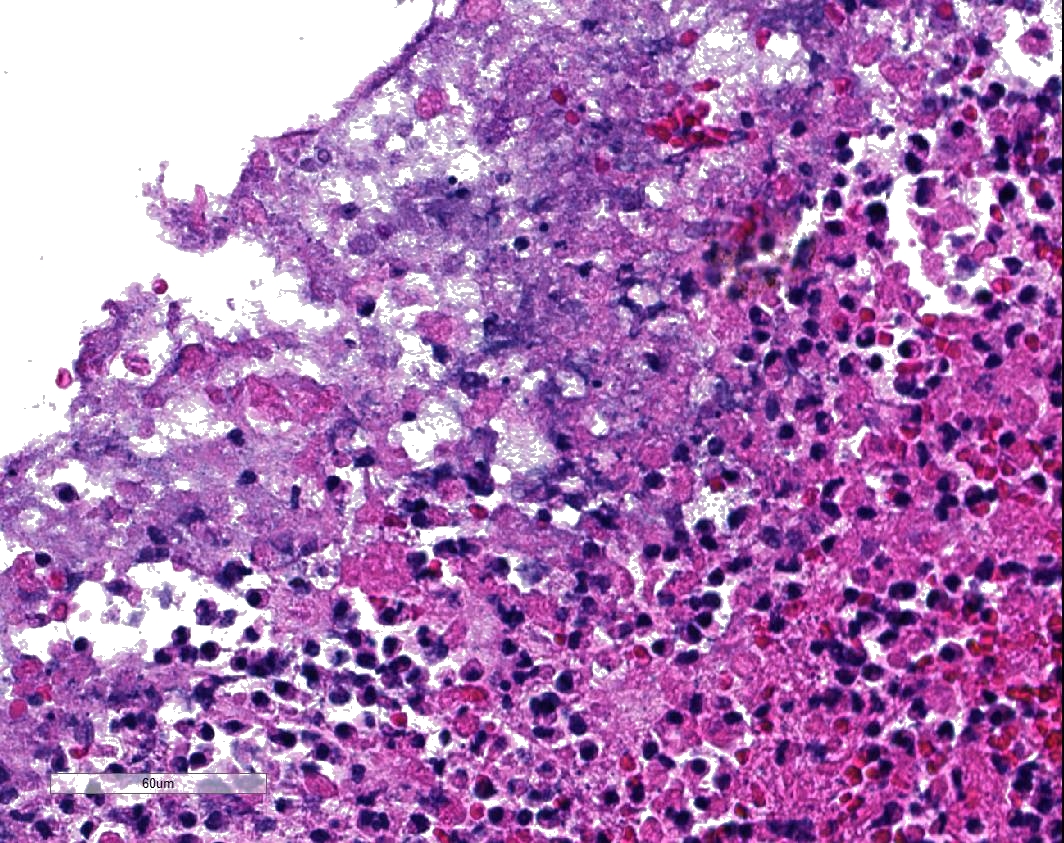
Microscopic Description: Two to three full-thickness sections of similarly affected cecum and colon are evaluated. Multifocally, there are "volcano lesions" composed of aggregations of sloughed epithelial cells, numerous degenerate and non-degenerate neutrophils, few erythrocytes, and fibrin within and extending from the underlying eroded epithelium and lamina propria into the lumen. Along the luminal aspect of these aggregates, frequent individual and clustered rod-shaped, straight pale basophilic bacteria up to 10 um long, occasionally with a visible clearing (oval spores), are admixed with cocci-shaped and shorter rod-shaped bacteria. The underlying epithelium is intact within some of these areas and partially to completely necrotic in others, with variable extension of inflammatory infiltrates into the mucosa. The superficial mucosa subjacent to some of these lesions is necrotic, with sparing of the remainder of the crypts. The lamina propria is moderately expanded by plasma cells, lymphocytes, and degenerate leukocytes. Necrosis extends full thickness overlying some of the underlying lymphoid follicles (Peyer?s patches/gastrointestinal-associated lymphoid tissue) within the sections of ileum and cecum. Small aggregates of fibrin are present within the lumina of a small to moderate number of the capillaries of the lamina propria and submucosa. The submucosa is multifocally expanded by small numbers of lymphocytes, plasma cells, and lower numbers of eosinophils, with mild to moderate increased submucosal clear space within some sections (edema). There is mild to moderate submucosal congestion.
Contributor Morphologic Diagnosis:
Cecum and colon, typhlocolitis, erosive and fibrino-necrotizing (pseudomembranous), multifocal, marked, acute
Contributor Comment: Clostridium difficile is a toxin-producing, gram-positive, spore-forming anaerobe best known for its role in causing pseudomembranous colitis in humans (often referred to as Clostridium difficile-associated disease or CDAD), often associated with antibiotic administration.5 As use of antibiotics has increased, so has reported cases of pseudomembranous colitis, with C. difficile acting as a source of a potent enterotoxin. To the author?s knowledge, Clostridium difficile-related pseudomembranous colitis with characteristic "volcano" lesions has not been described in New World monkeys, with a single reported case in a marmoset within the 2016 Wednesday Slide Conference that displayed more severe and diffuse histologic changes and a case report of several cotton-top tamarins that died suddenly following antibiotic therapy for Campylobacter spp. associated diarrhea, with identification of Clostridium difficile toxin within the feces in all and pseudomembranous colitis in two of the five described cases.8
In humans, several papers have described various morphologic stages of lesion in cases of pseudomembranous colitis, beginning with a focal epithelial necrosis with exudation of fibrin and neutrophils, followed by presence of a marked exudate extending through the area of mucosal ulceration and forming the classic "volcano" lesion, ultimately leading to a more diffuse and severe mucosal ulceration and necrosis with presence of a pseudomembrane.5,7,10 In this case, the volcano-like lesions were a prominent feature. Similar ?volcano lesions? have been described within piglets diagnosed with Clostridium difficile-associated typhlocolitis and within mice and hamsters and are reported rarely in horses.2,3,6
While CDAD is considered well-described in humans and has been characterized in several animal species, the challenges of definitive diagnosis are ongoing. Cultures of Clostridium difficile are capable of isolating the bacteria, but interpretation of results is not clear-cut, given the isolation of C. difficile in low numbers from asymptomatic animals that have not been treated with antibiotics and in higher numbers from asymptomatic animals following antibiotic treatment.2,13 For this reason, toxinotyping of cultured isolates is necessary to rule out presence of a non-toxigenic strain of C. difficile, though this is more readily available in human medicine than in veterinary medicine. Toxinotyping is a PCR-restriction fragment length polymorphism-based method that classifies strains of C. difficile based on variations in the pathogenicity locus (PaLoc), which is the region that codes for toxin A and toxin B, and groups strains by those with identical changes within the PaLoc region. Many isolates of C. difficile have been identified with varying toxigenic properties, with 34 toxinotypes reported based on sequence variations in the A and B toxin genes. PCR ribotyping allows further characterization of strains of C. difficile and their relatedness, which has been of particular interest due to geographical variation in the prevalence of various ribotypes.14
The primary virulence factors of C. difficile are two major exotoxins, toxin A and toxin B. Toxin A and Toxin B are both enterotoxins, while Toxin B is also a cytotoxin. Alone or together, these toxins have the ability to glycosylate and inactivate Ras GTPases, disabling key cell signaling pathways, and glycosylate Rho and interfere with its regulation of cytoskeletal actin.14 Fecal ELISA is commonly used to identify one or both of these toxins, and was utilized in this case to confirm the presence of Clostridium difficile A and B toxins in three of four other similarly affected monkeys within the same colony, though samples were not available for submission from this particular individual at the time of diagnosis.
Almost any antibiotic can cause disruption of the intestinal microbiota and subsequent Clostridium difficile infection, with clindamycin frequently implicated in human cases, along with other antibiotics such as cephalosporins and broad-spectrum penicillins that are widely prescribed.5 Resolution of clinical signs is often successful with vancomycin or metronidazole treatment.13 C. difficile infection has been more widely reported in the equine and implicated in gastrointestinal disease in the dog and cat, though there is controversy as to the importance of antibiotic exposure as a risk factor in development of CDAD in these species.2,12 Diet change and environmental stressors have also been implicated in disruption of the gastrointestinal flora and subsequent colonization and development of CDAD in several species.2,6
Contributing Institution:
University of Minnesota Department of Veterinary Population Medicine/Minnesota Veterinary Diagnostic Laboratory - https://www.vetmed.umn.edu/departments/veterinary-population-medicine ; https://www.vdl.umn.edu/
JPC Diagnosis: Cecum, colon (per contributor): Typhlocolitis, necrotizing, multifocal to coalescing, marked, with numerous extracellular bacilli.
JPC Comment: C. difficile has been the cause of disease in a wide variety of mammalian species. First identified from feces of clinically healthy human babies in the 1930s, the organism was originally named Bacillus difficilis because of the difficulties encountered in cultivating it. In humans, most infected people will remain asymptomatic, with the remainder developing variable GI signs ranging from watery diarrhea to pseudomembranous colitis1, particularly if they have been recently hospitalized or the recipient of antibiotics.
In humans, C. difficile
associated disease (CDAD) was always assumed to affect individuals of any age
except during the neonatal period, as it was thought that this specific group
may lack specific C. difficile toxin receptors. Although between 25 and 70%
of human neonates are colonized with C. difficile, these microorganisms
have been largely considered part of the commensal microbiota. Recently,
however, two 9- and 18- month-old children were diagnosed with CDAD, providing
evidence that C. difficile is a potential cause of bloody diarrhea in
neonates and young infants. In most animal species, CDAD is not age-dependent.
The exception to this are pigs, which are almost exclusively affected during
the neonatal period, up to approximately one week of age.11
The gut microbiota is the primary protection against C. difficile
overgrowth and overt disease. In addition to antibiotic administration,
alteration in bile acids (which promote the germination of C. difficile) has
been noted in affected human patients, adding another potential factor in CDAD.1
An emerging treatment for recurrent C. difficile infection is fecal
microbiota transplants, which showed significantly higher rates of resolution
of recurrent CDAD than those with conventional antibiotic treatment. In the
Netherlands, one large medical center has set up the ?Netherlands Donor Feces
Bank? and a panel of internal medicine and infectious disease specialists
review each case to ensure it fits a rigid set of inclusion criteria, accepting
80% of referrals and posting a 90% rate of success.1
In clinical disease, the bacillus is not infective. Mucosal necrosis and loss of barrier integrity is the result of liberation of a number of enzymes, including collagenase, hyaluronidase, and chondroitin-sulfatase, as well as the previously mentioned toxins, which act on the epithelial cell cytoskelton, leading to enterocyte disassociation, fluid loss, and local inflammation.1
C. difficile-associated disease (CDAD) affects a wide range of other mammals ? while always resulting in enterocolitis, its manifestation varies widely with the affected species. Table 1 summarizes the severity of disease in various species by enteron segment.
In rodents, CDAD is primarily cecal, resulting in ulcerative and rarely proliferative typhlitis and death. In pigs, the disease results in ulcerative typhitis or colitis with development of ?volcano ulcers?. An additional gross finding of mesocolonic edema4 (as well as diarrhea) makes CDAD a differential diagnosis for edema disease in swine.2 The difference is that C. difficile infections occurs in young piglets (1-7 days of age), while edema disease is a disease of weanling age pigs. It has been suggested as a pathogen for enteritis in young caves (Kach), as well as an agent that can cause necrotic enteritis in poultry (Uzal). In some Latin American countries, a linkage between high incidence in poultry and high prevalence of CDAD in humans has been identified.4 In rabbits, the lesion is primarily seen in the small intestine and concentrated in the ileum, often following antibiotic administration. It has also been reported sporadically in dogs, cats, ostriches, prairie dogs, and experimentally in non-human primates.11
1. Czaepiel J, Drozdz M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultariska D, Garlicki A, Biesiade. Clostridium difficile infection: a review. Europ J Clin Microbiol Inf Dis 2019; 38:1211-1221.
2. Diab, SS, Songer G, and Uzal, FA. Clostridium difficile infection in horses: A review. Veterinary Microbiology 2013;167:42-49.
3. Hutton ML, Mackin KE, Chakravorty A, and Lyres D. Small animal models for the study of Clostridium difficile disease pathogenesis. FEMS Microbiology Letters 2014;352(2):140-149.
4. Kachrimanidou, M, Tzika E, Filioussis. Clostidioides (Clostridium) difficile in food-producing animals, horses and hosuehod pets: a comprehensive review. Microorgainisms 2019; 7(12):667:doi 10.3390/microorganisms7120667.
5. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. New England Journal of Medicine. 1994; 330:257-262.
6. McElroy MC, Hill M, Moloney G, Aogain MM, McGettrick S, O?Doherty A, Rogers TR. Typhlocolitis associated with Clostridium difficile ribotypes 078 and 110 in neonatal piglets from a commercial Irish pig herd. Ir Vet J 2015; 69:10.
7. Price AB, Davies DR. Pseudomembranous colitis. J Clin Path 1977;30:1-12.
8. Rolland RM, Chalifoux LV, Snook SS, Ausman LM, Johnson LD. Five spontaneous deaths associated with Clostridium difficile in a colony of cotton-top tamarins (Saguinus Oedipus). Lab Anim Sci. 1997;47(5):472-476.
9. Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 6th ed. Vol 2. St. Louis, MO: Elsevier; 2016:183-183, 191-194.
10. Uzal FA, Navarro MA, Li J, Freedman JC, Shrestha A, McCline BA. Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe 2018; 53:11-20.
11. Weese, JS and Armstrong, J. Outbreak of Clostridium difficile-associated disease in a small animal veterinary teaching hospital. J Vet Intern Med 2003;17(6):813-816.
12. Zar FA, Bakkanagari SR, Moorthi KMLST, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clinical Infectious Diseases. 2007;45(3):302-307.
13. Zhu S, Zhang L, Zhang C, Chen X, Chen Q, Li Z. Comparison of polymerase chain reaction ribotyping, toxinotyping and nutritional aspects of toxin production of Clostridium difficile strains. Biomed Rep 2014;2(4):477-480.
14.
6. Uzal FA, Navarro MA, Li J, Freedman JC, Shrestha A, McCline BA. Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe 2018; 53:11-20.