CASE 4: S-000-2020 (JPC 4157768-00)
Signalment:
8 week old, female, Syrian hamster
History:
This Syrian hamster was on study for validation of a SARS-CoV-2 animal model. This animal was immunosuppressed through pretreatments with Cyclophosphamide. Although weight loss was noted clinically, the animal demonstrated signs of recovery and was euthanized thirteen days following challenge.
Gross Pathology:
At necropsy, lung lobes were speckled red with minimal amounts of fluid in the pleural cavity.
Laboratory results:
N/A
Microscopic description:
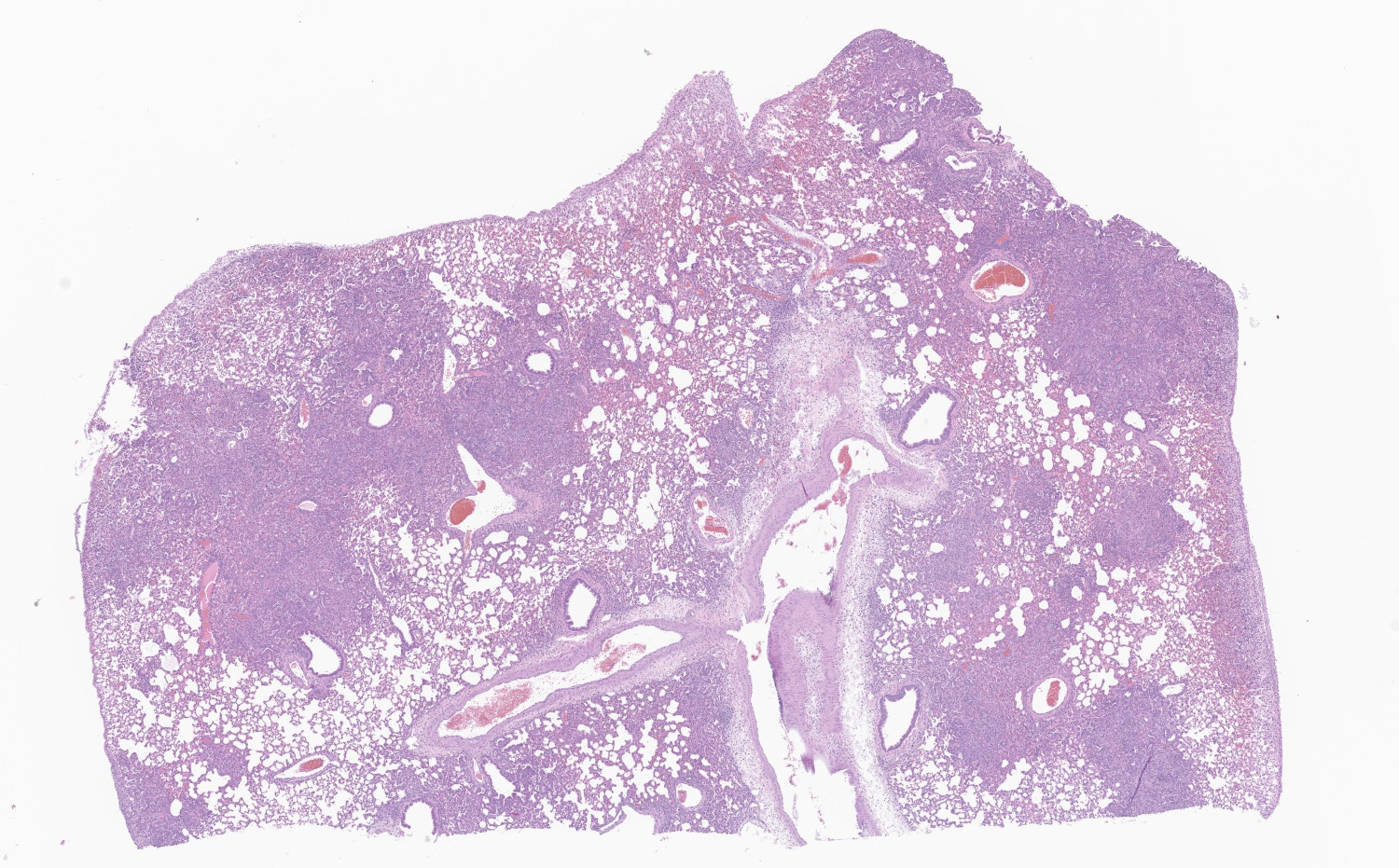
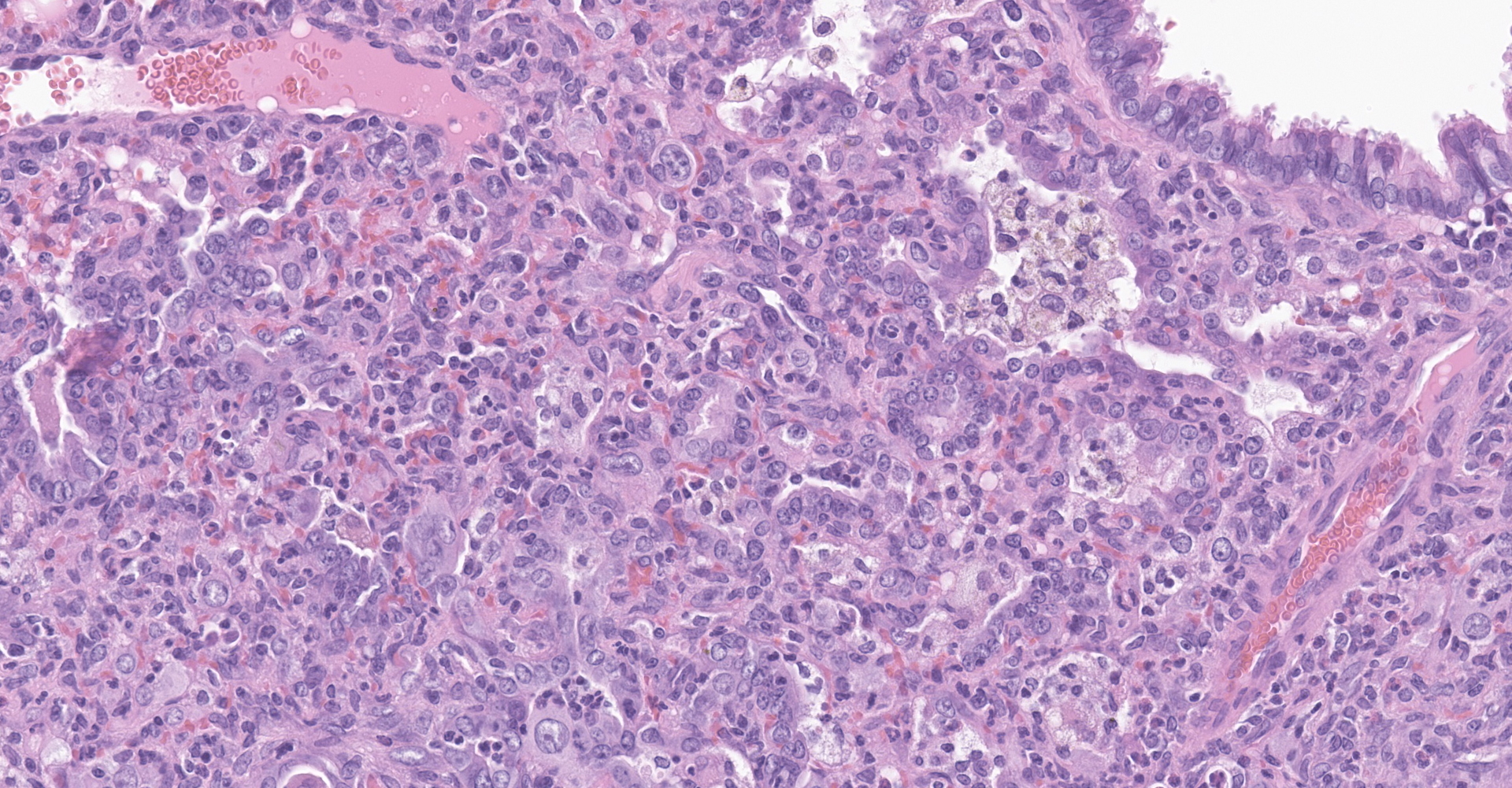
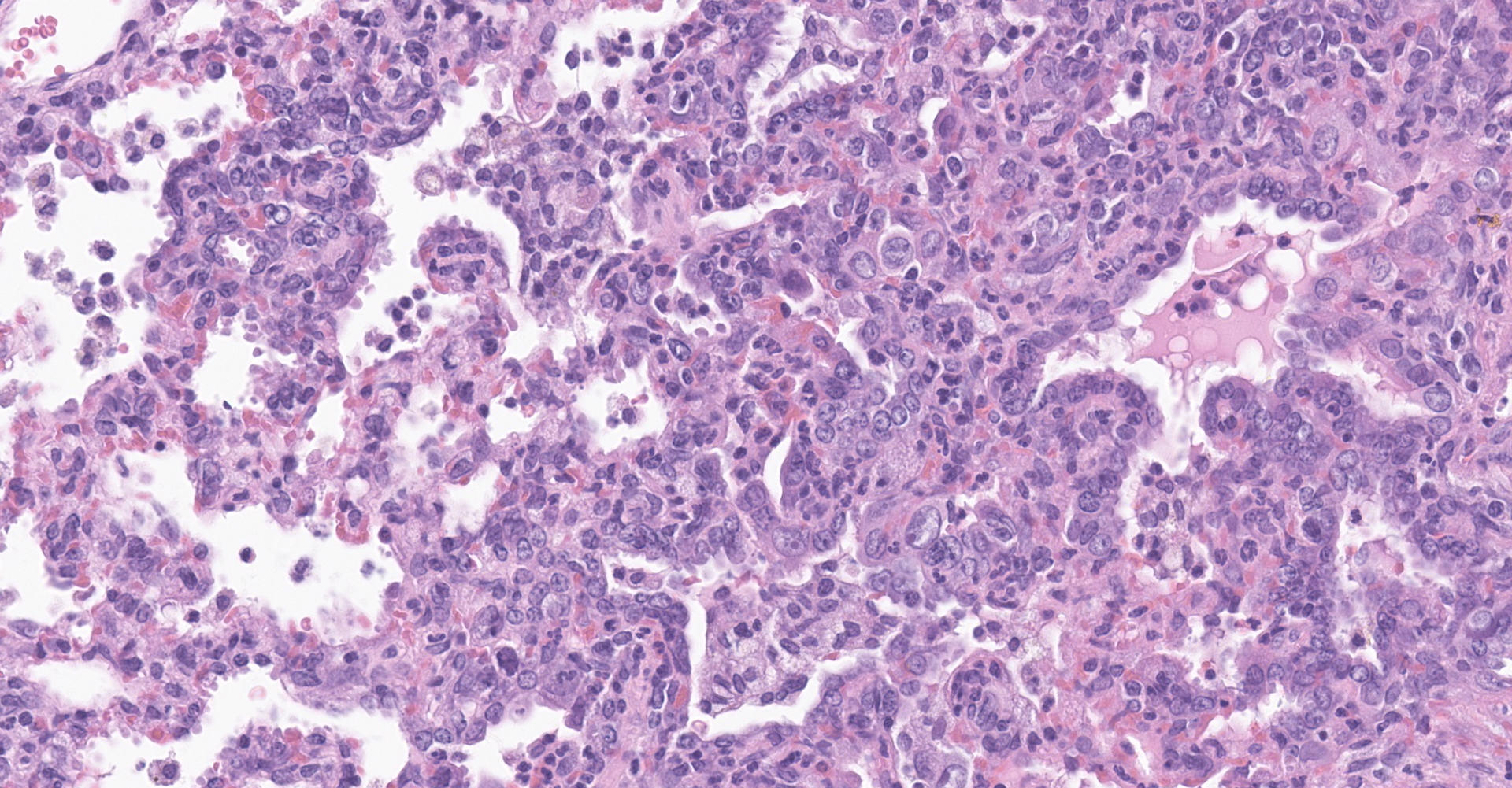
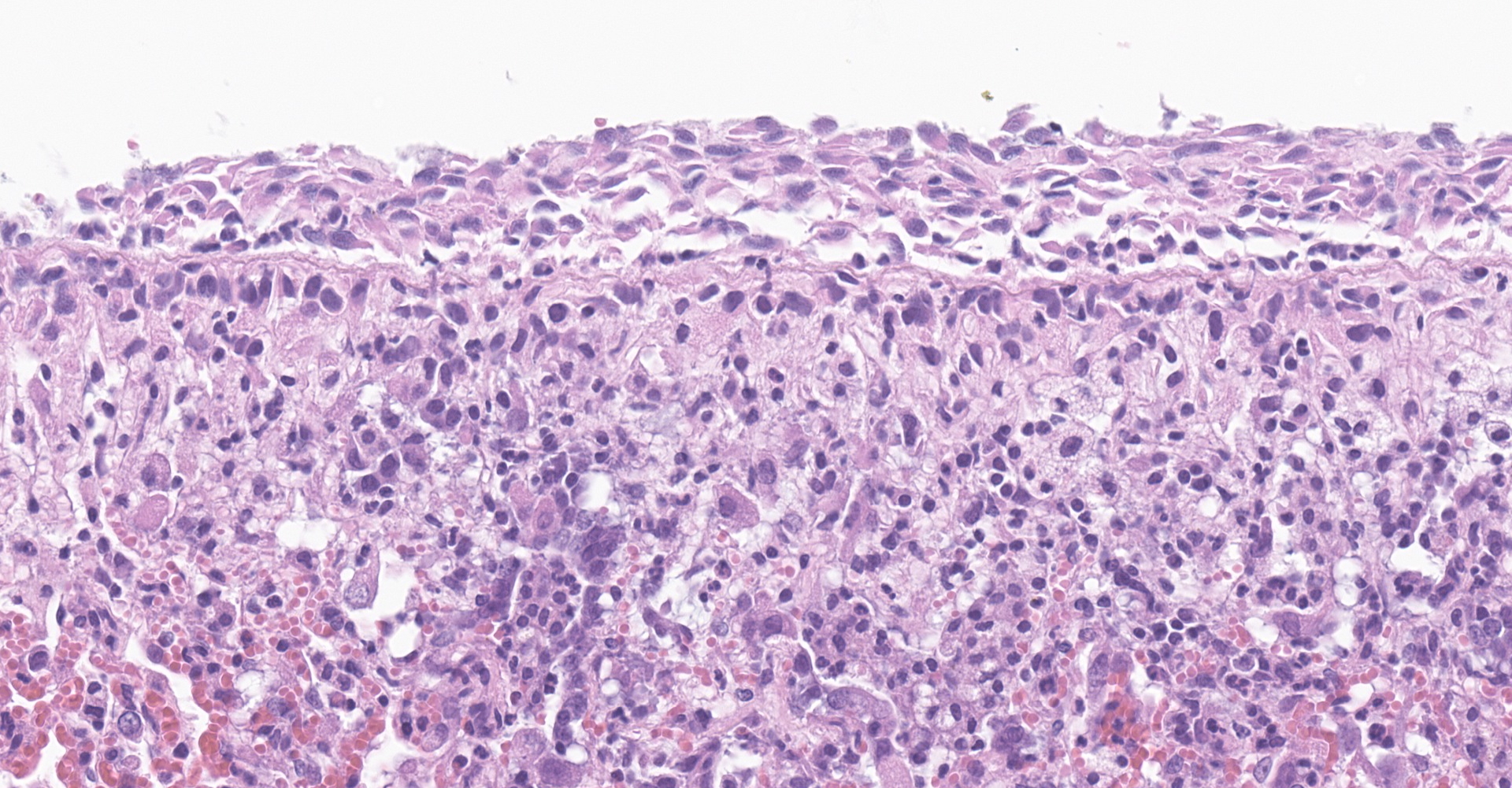
Lung: Affecting greater than 60% of the pulmonary parenchyma at all levels are multifocal to coalescing, random, and patchy areas of consolidation admixed with dense aggregates of macrophages, heterophils, fewer lymphocytes, multinucleate cells, necrotic cellular debris, hemorrhage, fibrin, and edema. Alveolar lumina contain swollen alveolar macrophages, previously mentioned inflammatory cells, hemorrhage, and necrotic debris. Within areas of consolidation alveolar septa are lined by palisading segments of low cuboidal epithelium (type II pneumocyte hyperplasia). Alveolar septa in these areas are expanded by inflammatory cells, fibrin, hemorrhage, and edema (septal necrosis). Inflammatory cells expand the peribronchial and peribronchiolar connective tissue with increased clear space (edema). Bronchial and bronchiolar epithelial cells exhibit the following: hyperplasia characterized by being bunched, stacked, and layered up to 4 cell layers thick, degeneration characterized by swelling, vacuolation and variable loss of cilia, and necrosis characterized by shrinking with a scant amount of hypereosinophilic cytoplasm and a pyknotic nucleus. The pleura is diffusely expanded up to 4x normal thickness by fibrous connective tissue, macrophages, heterophils, and lymphocytes. The pleural surface is lined by plump, reactive mesothelium (hypertrophy).
Contributor's morphologic diagnosis:
Lung: Pneumonia, bronchointerstitial, necrotizing, histiocytic, heterophilic, multifocal, marked with type II pneumocyte hyperplasia, hamster (Mesocricetus auratus), rodent.
Contributor's comment:
Coronaviruses are enveloped RNA viruses that can lead to intestinal and respiratory infections in humans and animals.8,15 A novel coronavirus infection identified as Coronavirus Disease 2019 (COVID-19) can result in severe and fatal pneumonia which was identified in patients from Wuhan City, Hubei Province, China in December 2019.19 The subsequent pandemic and dearth of knowledge regarding this emerging pathogen resulted in widespread research efforts to further characterize the pathogen, disease course, and potential animal models.
Attachment and entry of SARS-CoV-2 is mediated by the human angiotensin-converting enzyme 2 (ACE2) receptor which is expressed primarily in lung (airway epithelial cells and type II pneumocytes), intestine, kidney, and heart.7,18 Expression of ACE2 mRNA has also been described in the brain, testis, liver, spleen, bone marrow, thymus, lymph nodes, skin, and mucosa of the oral and nasal cavity.3 Histologic features of the disease in humans reflect that of SARS-CoV to include diffuse alveolar damage (DAD), hyaline membrane formation with multinucleate viral syncytial cells, and pulmonary edema.16
Since identification of the novel coronavirus, a number of studies have been conducted to establish an animal model to further characterize the disease and support therapeutic development efforts. At present, animal models include hamsters2,14, non-human primates11,12,17, ferrets4,13 and mice.1 In the present study, the most notable histologic lesions at 13 days post-challenge (dpc) were identified in nasal turbinates and lungs. There is olfactory mucosal erosion and/or ulceration with multifocal, olfactory epithelial necrosis that can be overlaid by a pseudomembrane of necrotic debris and degenerate inflammatory cells. Additional findings include moderate to marked submucosal edema and infiltration by heterophils and lymphocytes. The predominant finding in lungs were diffuse, bronchial to bronchiolar epithelial hyperplasia with a patchy, mosaic of pulmonary consolidation. Areas of consolidation contained dense aggregates of macrophages, heterophils, necrotic debris, type II pneumocyte hyperplasia, and multinucleate cells. Reported histopathologic lesions from hamsters infected with SARS-CoV-2 at earlier stages of infection include epithelial cell degeneration of the trachea, diffuse alveolar damage and hyaline membrane formation in the lungs, and congestion of nasal turbinate submucosa.2
Although not clearly identified for SARS-COV-2, multinucleate cells have been observed in SARS-CoV infection. Based on immunoreactivity with CD68, multinucleate cells have been identified as macrophage origin.6 The susceptibility of ferrets and cats are considered high, with dogs being of low susceptibility and pigs, chicken, and ducks being not susceptible.12 Reports of companion animal infection by SARS-CoV-2 have been reported, but data regarding post-mortem and microscopic findings in these species are limited. The significance of these findings in the context of zoonotic spread has yet to be determined and warrants further study.
Contributing Institution:
USAMRIID
https://www.usamriid.army.mil/
JPC diagnosis:
Lung: Pneumonia, bronchointerstitial, neutrophilic and histiocytic, multifocal to coalescing, subacute, severe, with marked Type II pneumocyte hyperplasia with syncytia formation, and multifocal septal necrosis.
JPC comment:
The contributor provides a concise summary on this case of SARS-CoV-2 in a hamster. A number of models of SARS-CoV-2 have been investigated, but serious natural disease has been experienced in mink farms.
The mink farming industry has recently received renewed attention due to the SARS coronavirus-2 (COVID-19) that has caused a worldwide pandemic and high rates of morbidity and mortality in human populations. Europe currently has an estimated 2750 mink farms, producing in excess of 27 million pelts annually. Due to the density of minks in group housing, transmissible disease often has ample opportunity to spread through farms. In early 2020, respiratory disease was noted in a number of mink farms in Denmark, which was isolated and confirmed to be SARS-CoV-2. In November 2020, the Ministry of Environment and Food of Denmark announced the culling of all mink in the country, due to concerns surrounding virus mutation. A number of coronaviral spike mutations had been noted in virus isolated from mink, leading to less effective neutralization by antibodies in a subset of humans that had been infected.5,10
SARS-CoV-2 has a similar presentation in mink as humans. Mink examined in the Netherlands had experienced respiratory disease, and an increase in mortality. Clinical signs also included watery to mucoid nasal exudates and anorexia as the disease progressed. Affected animals had grossly apparent interstitial pneumonia characterized by all lobes swollen, dark red, and did not collapse. Histologically, lungs had diffuse alveolar damage, type II pneumocyte hyperplasia, thickening of alveolar septa by fibrillar eosinophilic material and mononuclear cells, with alveolar exudate consisting of desquamated cells, mononuclear inflammatory cells, and low numbers of neutrophils. Bronchiolar epithelial cells were characterized by severe necrosis with syncytial cells. Pulmonary alveolar edema with abundant foamy alveolar macrophages, perivascular edema, and hyperemia of alveolar septa were also consistent findings. Immunohistochemistry demonstrated strong immunolabeling for SARS-CoV-2 in epithelial cells of the bronchi and bronchioles, alveolar epithelial cells, and desquamated pneumocytes and macrophages.9
References:
1. Bao L, Deng W, Huang B, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice [published online ahead of print, 2020 May 7]. Nature. 2020.
2. Chan JF, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility [published online ahead of print, 2020 Mar 26]. Clin Infect Dis. 2020
3. Jin Y, Yang H, Ji W, et al. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):372. Published 2020 Mar 27.
4. Kim YI, Kim SG, Kim SM, et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe. 2020;27(5):704-709.e2.
5. Koopmans M. SARS-CoV-2 and the human-animal interface: outbreaks on mink farms. Lancet Infect Dis. 2021; 21(1):18-19.
6. Kuiken T, Fouchier RA, Schutten M, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362(9380):263-270.
7. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565-574.
8. Masters, PS, and Perlman S. (2013) Coronaviridae. In Fields Virology. pp. 825-858.
9. Molenaar RJ, Vreman S, Honing RWHD, et al. Clinical and Pathological Findings in SARS-CoC-2 Disease Outbreaks in Farmed Mink (Neovison vison). Vet Path. 2020; 57(5):653-657.
10. Munnink BBO, Sikkema RS, Nieuwenhuije DF, et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021; 371(6525):172-177.
11. Munster VJ, Feldmann F, Williamson BN, et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2 [published online ahead of print, 2020 May 12]. Nature. 2020.
12. Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368(6494):1012-1015.
13. Shi J, Wen Z, Zhong G, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016-1020.
14. Sia SF, Yan LM, Chin AWH, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters [published online ahead of print, 2020 May 14]. Nature. 2020;10.1038
15. Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res 2011;81:85-164.
16. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [published correction appears in Lancet Respir Med. 2020 Feb 25;:]. Lancet Respir Med. 2020;8(4):420-422.
17. Yu P, Qi F, Xu Y, et al. Age-related rhesus macaque models of COVID-19. Animal Model Exp Med. 2020;3(1):93-97.
18. Zemlin AE, Wiese OJ. Coronavirus disease 2019 (COVID-19) and the renin-angiotensin system: A closer look at angiotensin-converting enzyme 2 (ACE2) [published online ahead of print, 2020 Jun 2]. Ann Clin Biochem. 2020.
19. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733.