Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 25
6 May, 2020
CASE IV: 25681 (JPC 4115969).
Signalment: 2 year old neutered male American Quarter Horse (Equus ferus caballus)
History: The horse had a history of muscle wasting and pyrexia that did not respond to corticosteroids or NSAIDS. There was ultrasonographic evidence of mineralization of multiple internal organs that led to euthanasia.
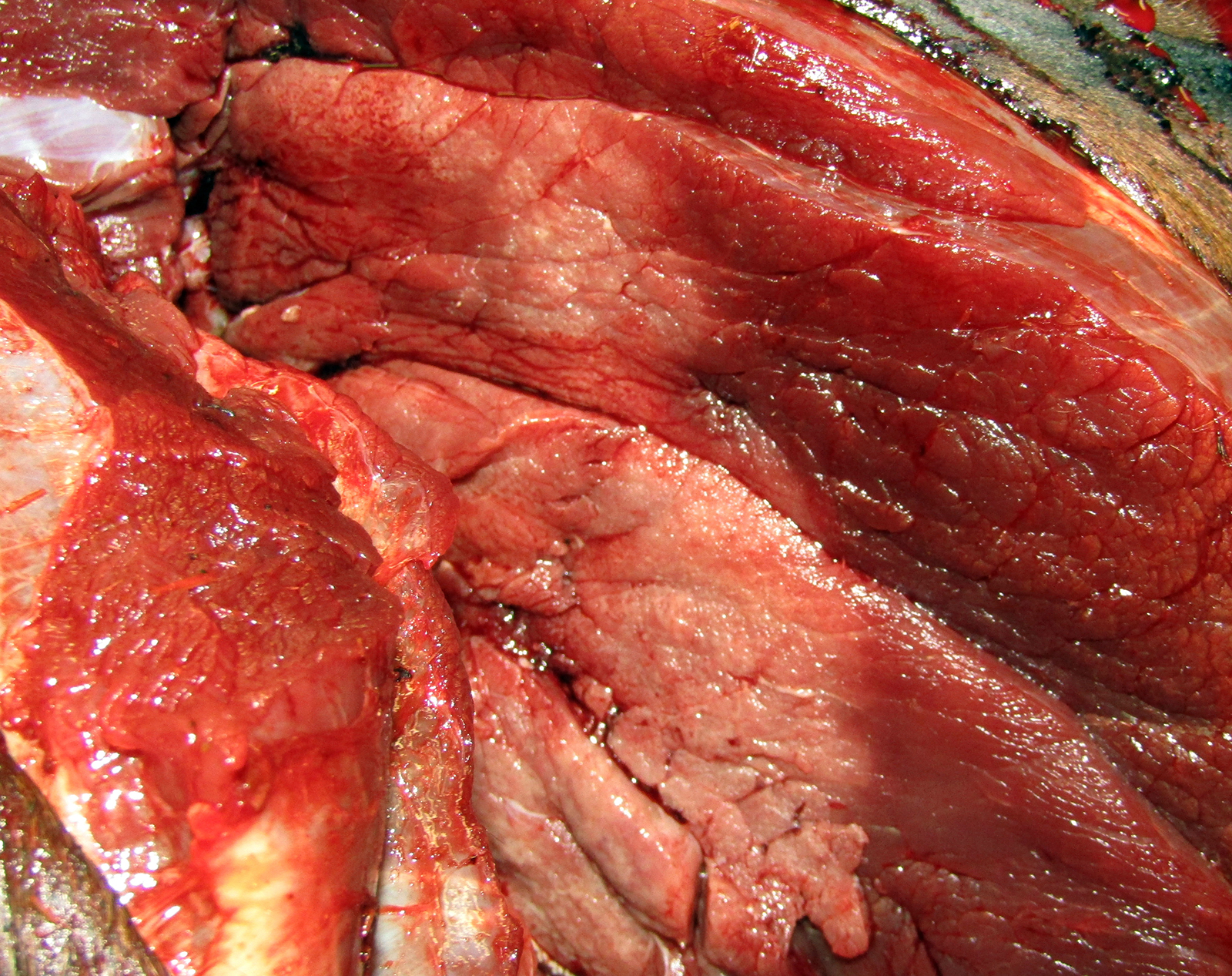
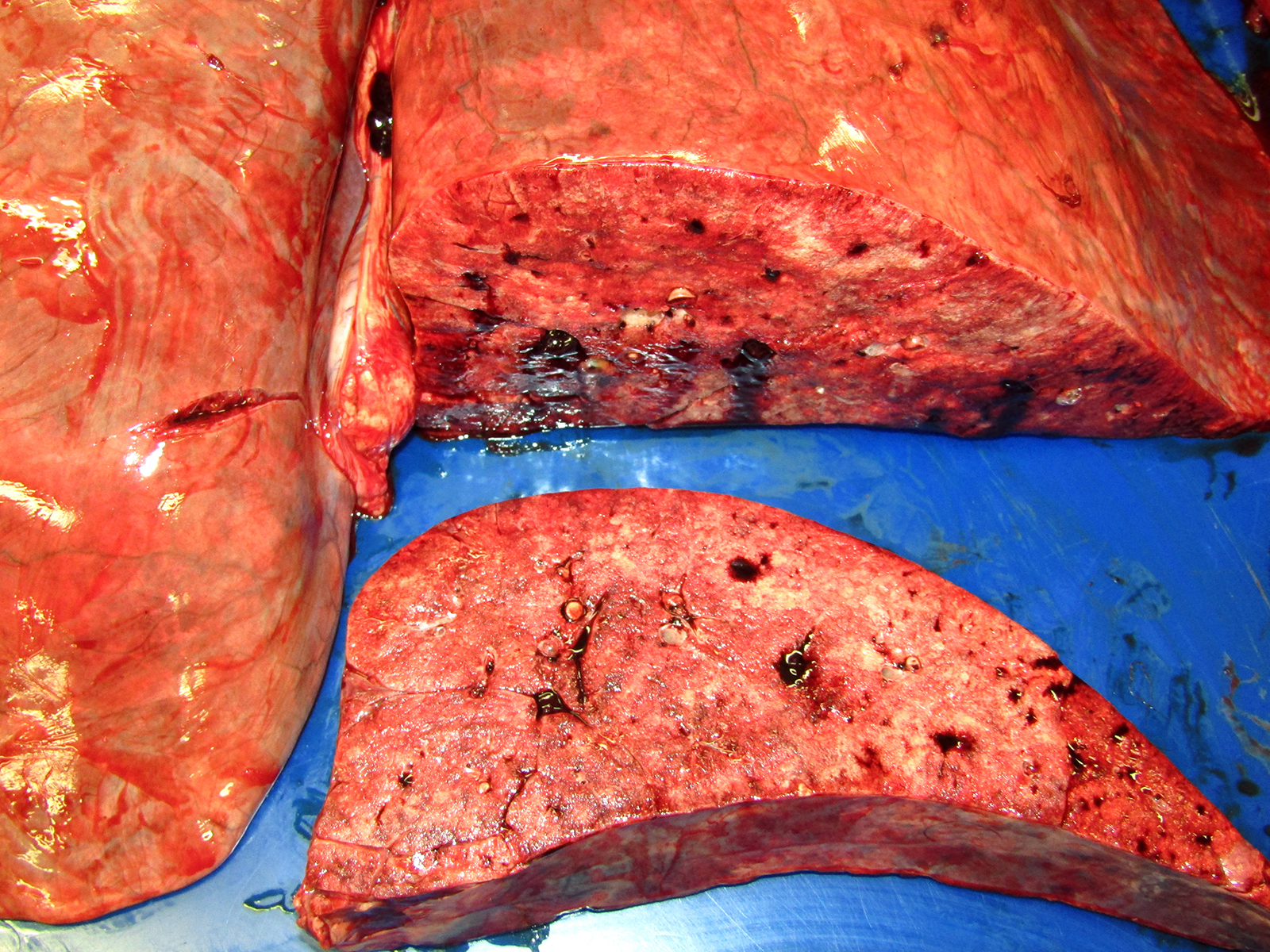
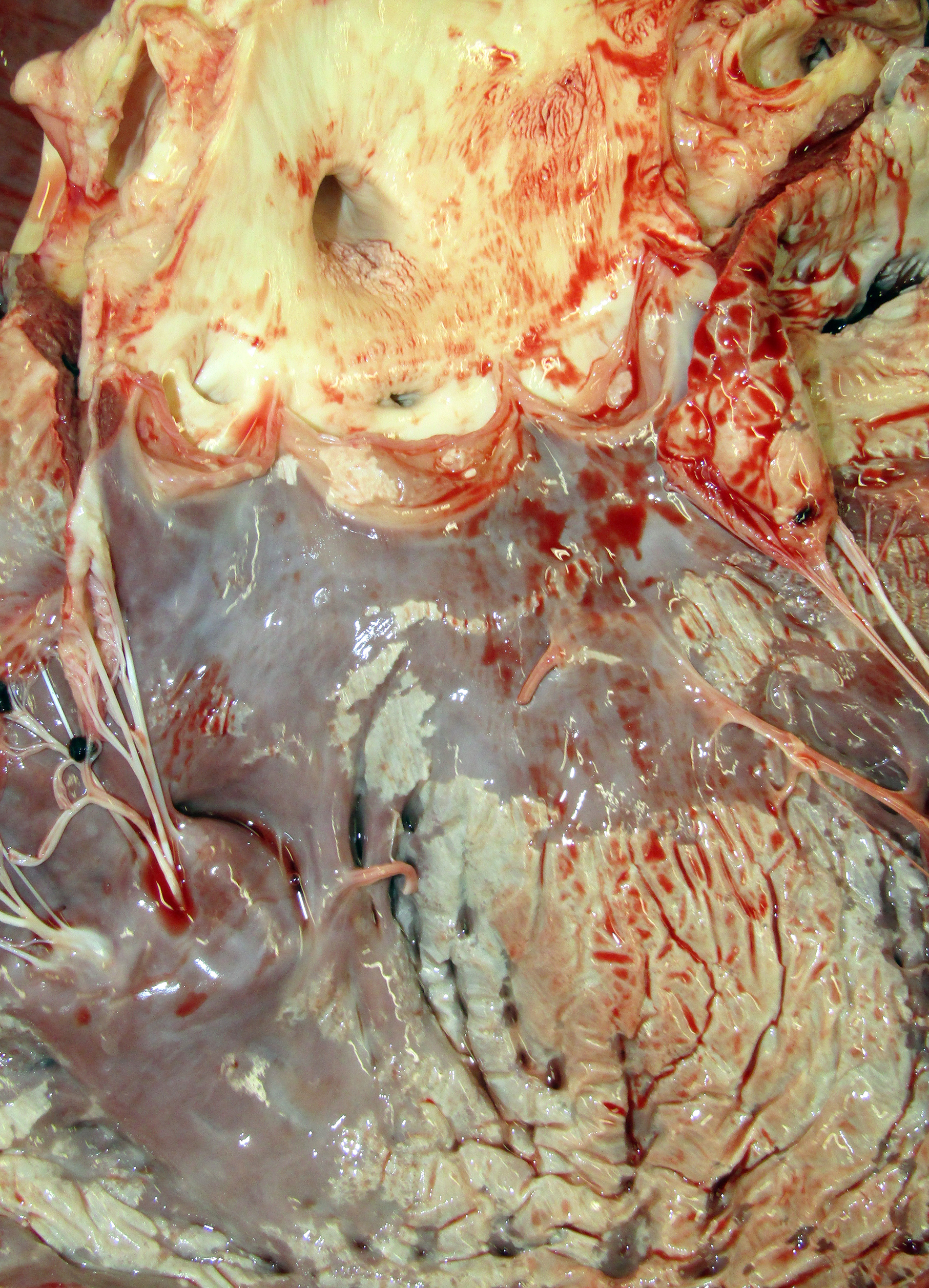
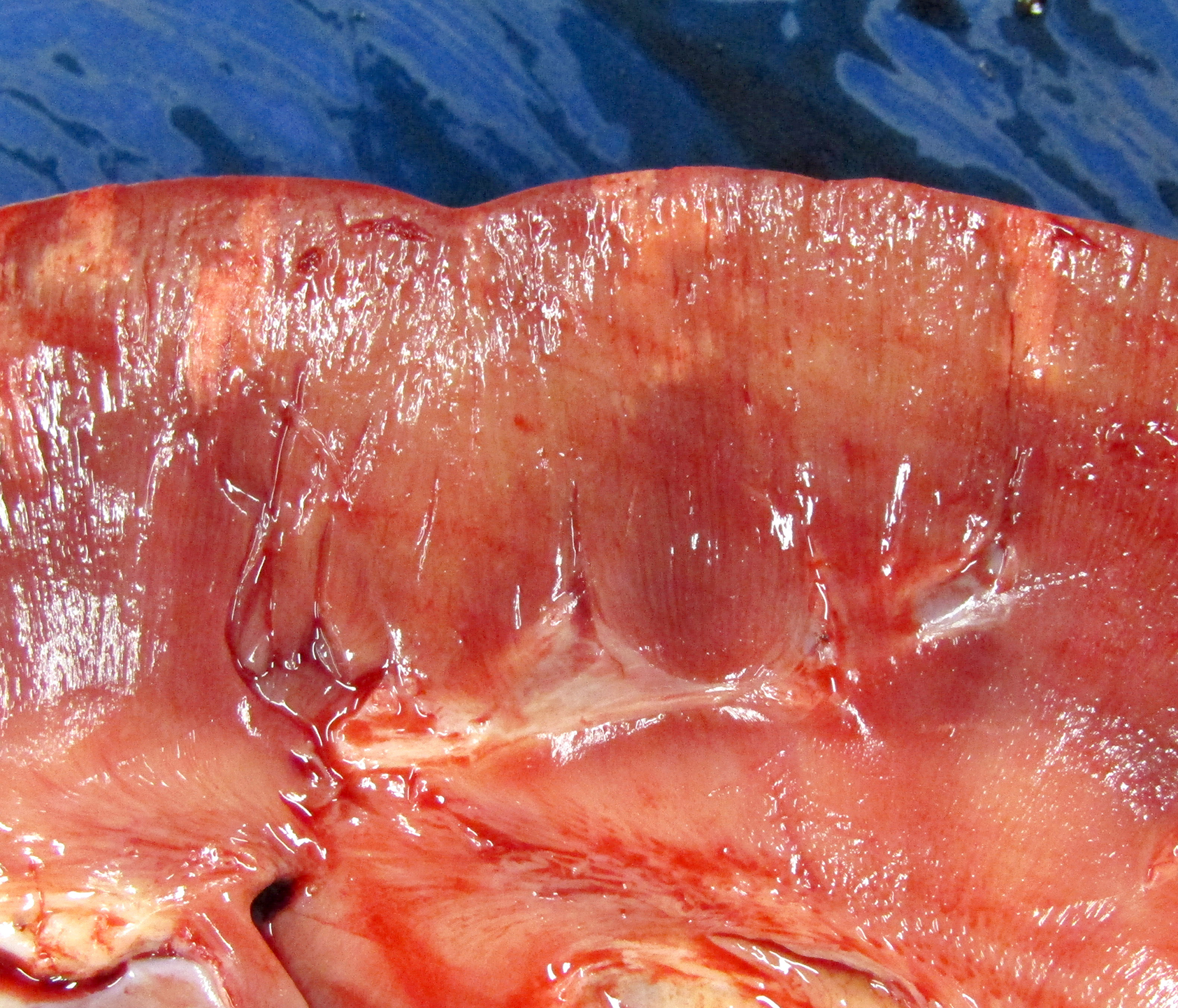
Gross Pathology: The horse weighed 406 kg and was fair body condition. Skeletal muscle of the gluteal and biceps groups of both rear limbs contained patchy pale areas (Fig. 1). Tongue and cardiac muscle are similarly affected. The lungs failed to collapse, even after incision, and were firm and somewhat gritty (Fig 2). Patches of endocardium had a wrinkled appearance, was hard and had a pale gray color (Fig 3). Both kidneys had multiple wedge-shaped, pale, gritty cortical foci (Fig 3). Petechial hemorrhages and erosions were found in the glandular stomach.
Laboratory results:
|
Test |
December 7 |
December 16 |
Reference |
|
WBC |
15.44 X 103 /ul ? |
21.76 X 103 /ul ? |
5.40-14.3 X 103 /ul |
|
Segs |
13.74 X 103 /ul ? |
17.41 X 103 /ul ? |
2.26-8.85 X 103 /ul |
|
Fibrinogen |
0.4 gm/dL |
0.6 gm/dL ? |
0.1-0.4 gm/dL |
|
Urea Nitrogen |
21 mg/dL |
39 mg/dL ? |
11-24 mg/dL |
|
Creatinine |
0.9 mg/dL |
2.1 mg/dL ? |
0.9-1.7 mg/dL |
|
Cl- |
96 meq/L |
94 meq/L |
95-105 meq/L |
|
Calcium |
11.5 mg/dL |
11.1 mg/dL |
11.0-12.9 mg/dL |
|
Phosphorus |
4.1 mg/dL ? |
8.7 mg/dL ? |
1.8-2.1 mg/dL |
|
Triglyceride |
58 mg/dL ? |
84 mg/dL ? |
14-62 mg/dL |
|
AST |
>10,000 U/L ? |
5324 U/L ? |
203-415 U/L |
|
CK |
>80,000 U/L ? |
6136 U/L ? |
112-496 U/L |
|
Urine 2/07/18 |
Brown, opaque, pH 9.0 |
|
|
|
Heme |
3+ |
|
|
|
Urine specific gravity |
1.063 |
|
|
|
Urine Sediment Cytology |
Marked amorphous material |
|
|
|
Strep equi SEM ELISA |
Moderately positive |
|
|
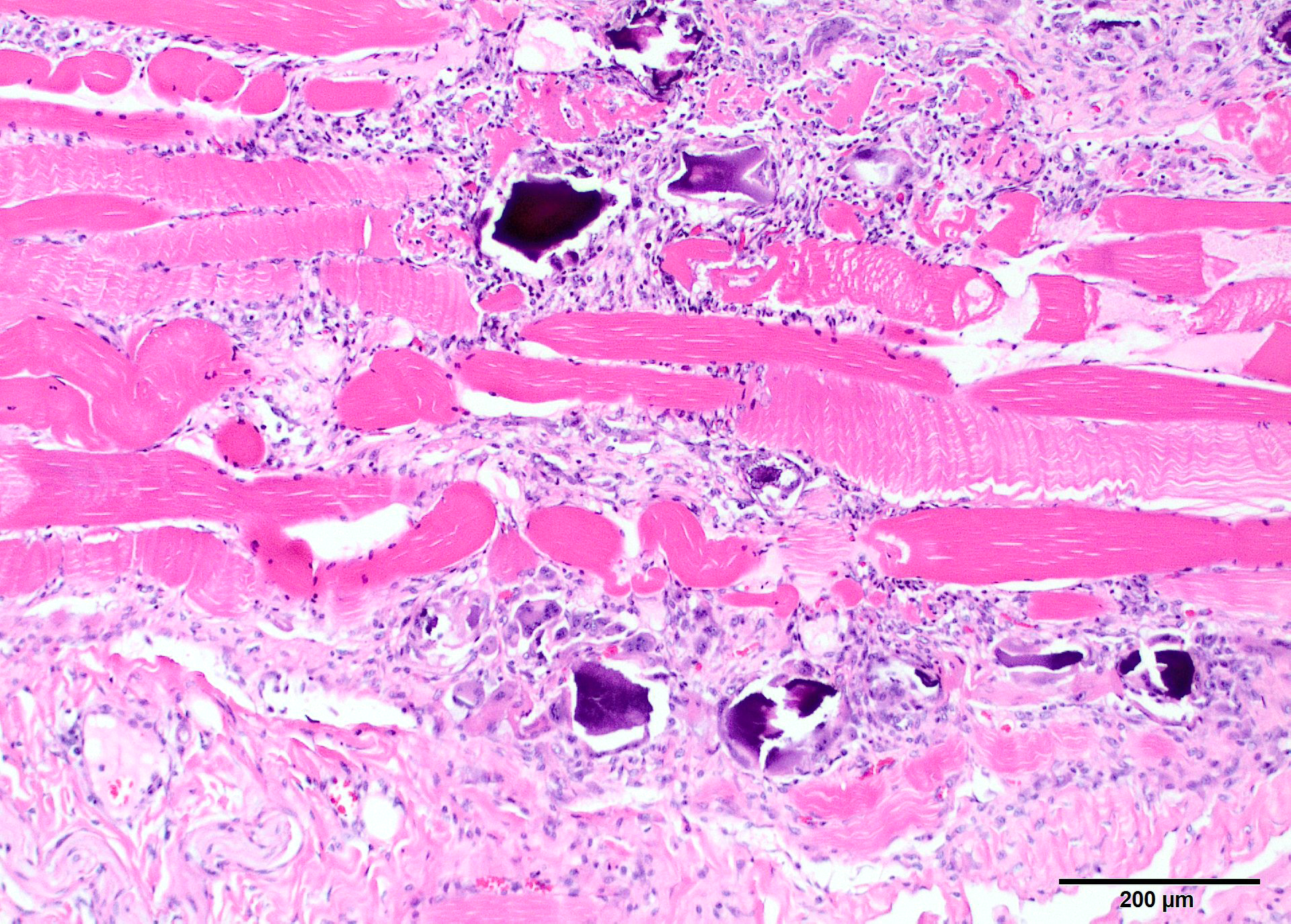
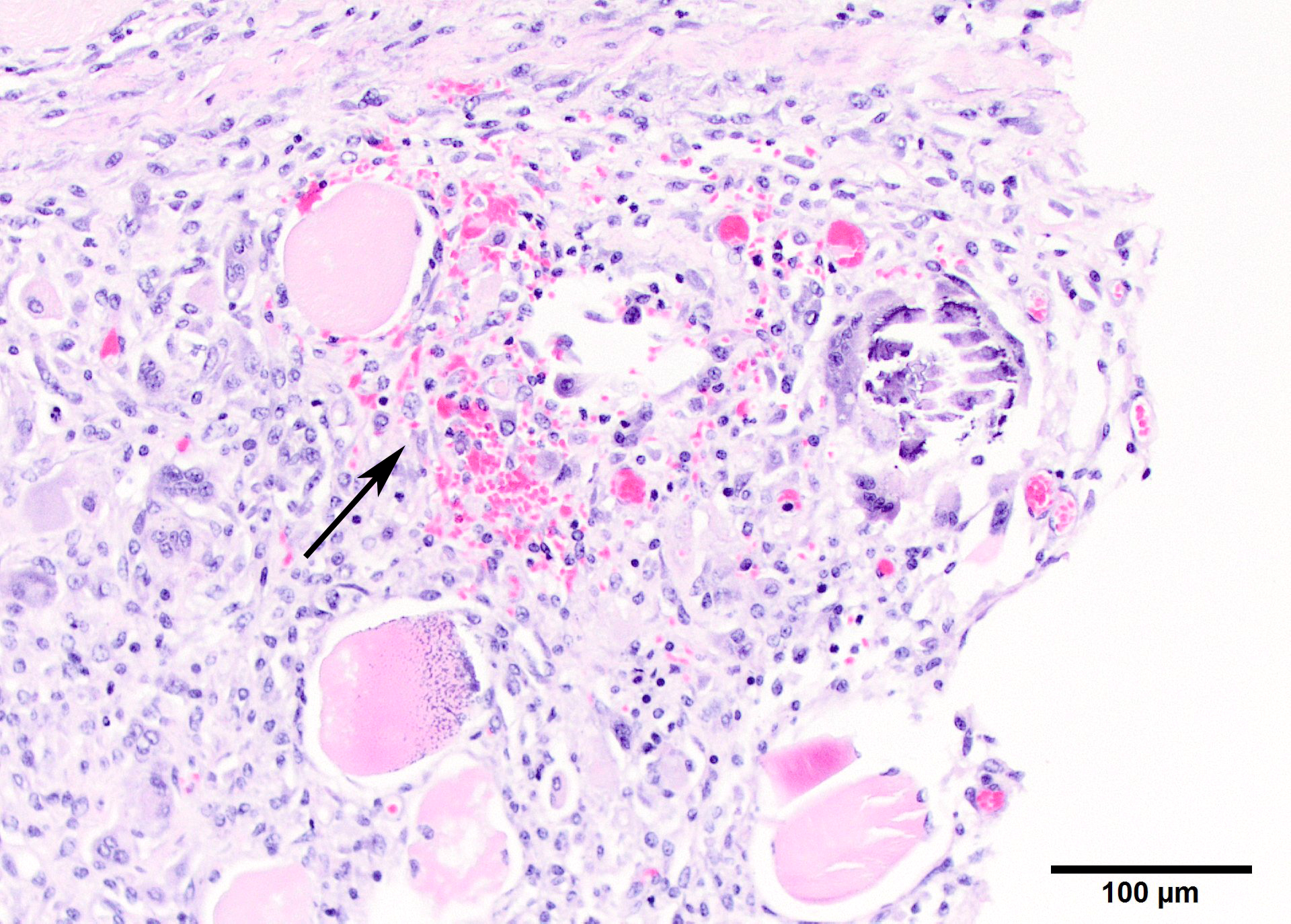
Microscopic Description: Two sections of muscle are submitted, one minimally and one extensively affected (gluteal). There is multifocal, degeneration, necrosis and mineralization involving single or clustered myofibers. Associated with degenerating fibers are infiltrating macrophages, multi-nucleate giant cells, lymphocytes and neutrophils Foci of degenerate fibers are associated with mild to moderate interstitial fibrosis. Multinucleate regenerating muscle fibers have lightly basophilic nuclei, and central nuclei are present. Small to medium-sized arterioles in affected areas are characterized by coagulative necrosis and mineralization affecting the tunica intima and tunica media (Figs. 5,6).
Other microscopic diagnoses (not found on the tissues submitted) include mineralization of alveolar walls and arteries of the lung, mineralization of the epicardium, endocardium and muscle of the heart and aorta, and multifocal mineralization of the renal cortex, gastric mucosa and tongue muscle.
Contributor Morphologic Diagnosis:
Chronic-active necrotizing myodegeneration with myofiber mineralization and attempted muscle regeneration
Contributor Comment: This animal has characteristic lesions in muscle and other organs consistent with systemic calcinosis.3,5,8 Skeletal muscles affected by this condition undergo gross atrophy. Large muscles, especially the gluteal, have more severe and extensive lesions, and the more severely affected specimen submitted is gluteal muscle. Other muscles can be much less affected. Histologic lesions are severe muscle necrosis. Necrosis in this case is associated with acute contraction bands, sarcoplasmic vacuolation, loss of striations and dystrophic calcification. Foci involving multiple fibers within a single muscle appear to be random, and segmental. Involvement of multiple fibers in one spot, in association with vascular mineralization suggests that vascular damage may be influence lesion distribution. Macrophage infiltrate and regenerative changes are often concurrently present in foci where multiple fibers are involved. Acute and chronic damage exists side-by-side, although the regeneration does not appear to be conspicuously successful.
Systemic calcinosis is an invariably fatal multi-systemic disease of horses that is thought to be a manifestation of calciphylaxis. Caliciphylaxis, or Systemic Uremic Arteriopathy in humans most often occurs in advanced uremia, prolonged dialysis, and renal transplantation. Muscle manifestations can be present, may be the presenting complaint,4,6 and are manifest as a subacute proximal myopathy. Cutaneous calcification is the most common site of calcium deposition in people, along with calcification of multiple internal organs. Patients also have elevated serum phosphate and normal serum calcium.
The equine disease affects younger horses, primarily Quarter horses and Paint horses. Tissue mineralization is multisystemic, and clinical signs relate to the severity of mineral deposition in various organs. This animal presented with muscle weakness and ataxia, and the muscular system is usually involved. In this horse, mineralization of the lung and heart was also severe, even though the horse presented with signs of lameness. Numerous organs can be affected, including the liver and intestine in the analogous human disease. Only a handful of equine cases have been reported.
The equine disease may be immune-mediated, with some similarities to dermatomyositis, but there not yet immunologic support for this hypothesis. In some humans, the presence of suspected lupus was found in one patient,6 and vascular complement deposition has been found in another patient, providing potential support for this idea.1
Mineralization of soft tissue may be dystrophic (deposition of mineral on dead tissue) or metastatic (deposition of mineral on otherwise normal tissue).
Muscle calcification can occur without vascular damage. Direct sarcolemmal damage causes contraction bands in muscle fibers. Calcium entry into muscle fibers eventually results in mitochondrial overload. Mineralization is a hyperacute event that is virtually concurrent muscle degradation by calpains.2
However, dystrophic mechanisms are more likely in calciphylaxis due to the occurrence of vascular mineralization and thrombosis that is associated with calcification of myofibers. This multifocal distribution of muscle lesions that contain more to less acute damage also suggests that arteriolar damage is primary. Calcification can develop on otherwise normal tissue (metastatic) when the Ca X P product exceeds 65. In this patient, the Ca X P product was 47 on a first chemistry and 97 on a second. Calcification may be a result of RANK-l and TNF activation of osteoclasts, and hyperphosphatemia. The mechanism is thought to be through parathyroid hormone release. Vitamin D can also produce hyperphosphatemia, which usually results in hypercalcemia; serum calcium was within normal limits in this horse and the amplified Ca X P product was primarily due to hyperphosphatemia. Attempts to measure parathyroid hormone in the patient were unsuccessful due to prolonged sample storage.
In people, administration of parathyroid hormone and corticosteroid exacerbates the calciphylaxis and leads to death. It is argued that arteriolar smooth muscle is not normal in uremic patients,7 and uremic patients without calciphylaxis have vascular mineralization anyway. In any event, since corticosteroids are a mainstay of therapy for myositis in veterinary medicine, misdiagnosis is unlikely to improve clinical outcome.
Contributing Institution:
Veterinary Medical Diagnostic Lab
University of Missouri
www.vmdl.missouri.edu
JPC Diagnosis: Skeletal muscle: Myositis, necrotizing and granulomatous, polyphasic, diffuse, severe, with mineralization.
JPC Comment The contributor has provided an excellent review of systemic
calcinosis in the horse, as well as cellular mechanisms of tissue
calcification. In this particular specimen, the wide range of changes in
affected myofibers (ranging from acute swelling and hyalinization, to
fragmentation and mineralization) indicates a polyphasic lesion, one that
occurs over time. In monophasic lesions, such as may be seen with acute
myopathic toxic injury, such as ionophore toxicosis, skeletal muscle lesions
are approximately at the identical stage or degeneration or necrosis.3
A wide range of conditions can result in local or systemic muscular
calcification in horse, both dystrophic and metastatic. Traumatic damage to
myofibers, such as may be seen with injections, will result in monophasic
dystrophic calcification. A range of plant toxicoses may result in either
monophasic lesions (in acute overwhelming intoxications) or polyphasic lesions
(which are more common, in prolonged grazing). Toxicosis with sublethal doses
of ionophores may result in monophasic lesions with mineralization of effete
myofibers. Some species of Cassia result in monophasic skeletal muscle
lesions in horses and ruminants; recumbent animals often do not recover. A
number of plants including Cestrum diurnum, Trisetum flavescens,
and plants of the genus Solanum accumulate analogs of activated Vitamin
D, which result in excessive absorption of calcium from the intestine and
subsequent metastatic calcification of skeletal muscle and many other organs. These
lesions are polyphasic and progressive, and skeletal muscle mineralization is
considered to be one of the earliest affected sites. Vitamin E selenium
imbalance may result in significant polyphasic damage to myocardial and
skeletal muscle (particularly the muscles of deglutition and the neck and
shoulder muscles) in foals, and less severe lesions within the skeletal muscles
in adult horses. Recurrent non-lethal cases of exertional myopathies may
present as polyphasic lesions.3
References:
1. Aouizerate J, Valleyrie-Allanore L, Limal N, et al. Ischemic myopathy revealing systemic calciphylaxis. Muscle & Nerve. 2017;56:529-533.
2. Cooper BJ, Valentine B.Muscle and Tendon. In: Maxie MG, ed. In: Pathology of Domestic Animals, vol.1. 6th ed. St. Louis:Elsdevier; 2017:161-162.
3. Durwood-Akhurst SA, Valberg SJ. Immune-mediated muscle diseases of the horse. Vet Pathol. 2018;55:68-75.
4. Eldelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as painful, proximal myopathy. Postgrad Med J. 1992;68:201-211.
5. Fales-William A, Sponseller B, Flaherty H. Idiopathic arterial medial calcification of the thoracic arteries in an adult horse. J Vet Diagn Invest. 2008;20:692-697.
6. Randall DP, Fisher MA, Thomas C. Rhabdomyolysis as the presenting manifestation of calciphylaxis. Muscle & Nerve. 2000;23:289-293.
7. Rogers NM, Teubner DJO, Coates TH. Calcific uremic arteriopathy: advances in pathogenesis and treatment. Semin Dialysis. 2007;20:150-167.
8. Tan, J-Y, Valbberg SJ, Sebastian MM, et al. Suspected systemic calcinosis and calciphylaxis in 5 horses. Can Vet J. 2010;51:993-999.