Signalment:
Gross Description:
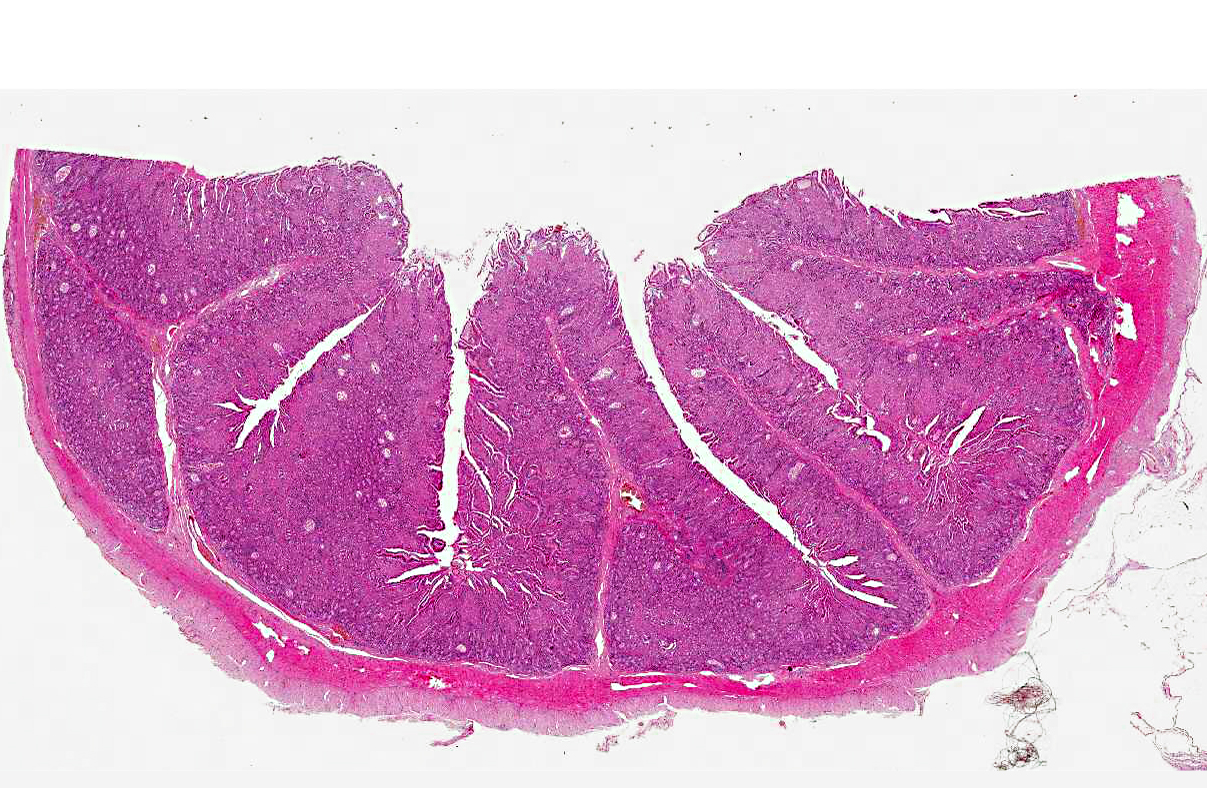
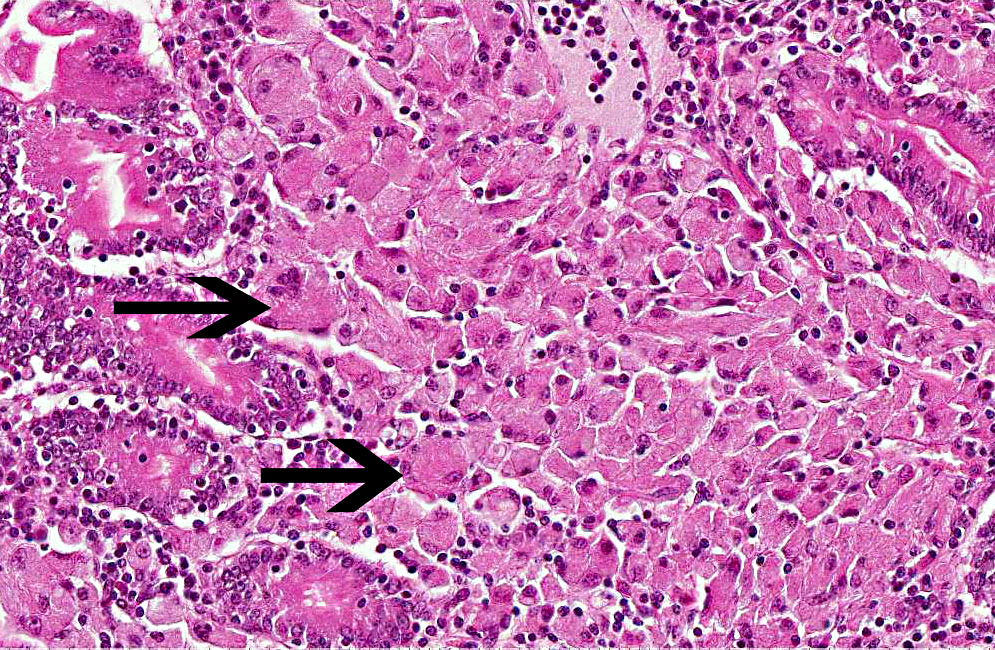
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Approximately 30% of the cows of 24 months and older tested positive for paratuberculosis and only one heifer was BVD positive, persistently infected (viremic animal without antibodies). All the other tested animals were positive for BVD antibodies (according with a high viral environmental circulation). After these results, the BVD positive cow was slaughtered. Vaccination against BVD was performed in the farm.Â
Ziehl-Nielsen stain demonstrated numerous acid-fast bacilli in the cytoplasm of macrophages.Â
Condition:
Contributor Comment:
The etiologic agent of Johne's disease has been reduced to the subspecies status within M. avium on the basis of the high (>90%) DNA homology among typical paratuberculosis strains and type strains of M. a. avium. The Johne's disease agent, which in culture is slow-growing and dependent on mycobactin as a source of iron, possesses some unique cultural and biochemical traits. Genetically, a distinct difference from M. a. avium is the presence of the insertion sequence, IS900, of which M. a. paratuberculosis has 15-20 copies per organism.
Paratuberculosis is a chronic wasting enteritis that affects mainly domestic and wild ruminants worldwide but occurs also in camelids, lagomorphs, rodents, carnivores, birds and rarely in equids. Infection can also be experimentally reproduced in pigs, rabbits, mice, hamsters and primates.(2,4)
Mycobacteria are shed in feces, and the main route of infection is fecal-oral, but microorganisms are shed in milk, semen and urine and are able to cross the placental barrier. Infection occurs most commonly during the first days of life, when MAPS is ingested via colostrum or by contaminated foodstuff. Calves are especially prone to the infection due to the lack of a functional hematointestinal barrier. After ingestion MAPS binds to the luminal surface of M cells and reaches (through phagosomes) Peyers patches where it can be phagocytosed by tissue macrophages. The virulence attributes of M. a. paratuberculosis are poorly understood, but presumably reside in resistance to killing in macrophages, through inhibition of the conversion of phagosomes to phagolysosomes. MAPS are highly adapted for survival within bovine mononuclear phagocytes.(15) The organisms proliferate in cytoplasmic vacuoles, and transmit to adjacent macrophages, expanding the population of infected cells and recruiting elements of the humoral and cell-mediated immune system to the lesional tissue. Both IL-14 dependent and IL-14 independent mechanisms appear to be involved in attenuation of phagosome acidification and phagolysosome fusion.(16) Mycobacteria acquire iron from ferritin stored in macrophages and, since the availability of iron is greatest in tissue macrophages of ileocecal junction, this is the site with initial and more severe lesions characterized by a diffuse granulomatous enteritis. Infected tissue macrophages spread via leukocyte trafficking in afferent lymphatic vessels to ileocecal lymph nodes leading also to a granulomatous lymphadenitis.(4,16)
Chronic, continuous or intermittent, often intractable, diarrhea, and emaciation are the most common and are associated with severe milk drop and reduced fertility.(16) Low total protein as a consequence of protein malabsorption and loss(2) is the most common clinical chemistry abnormality associated with low albumin serum while globulin values are usually unaffected.(14)
Specific gross lesions occur in the intestine and regional lymph nodes. The classical intestinal change is diffuse thickening of the mucosa, which is folded into transverse rugae, the crests of which may be congested. When well developed, the mucosal folds cannot be smoothed out by stretching. The intestinal serosa often has a slight granular and diffusely opaque appearance and foci of ulceration may be present. The mesenteric nodes, particularly the ileocecocolic, are always enlarged, pale, and edematous, especially in the medulla. Lymphangitis is the most frequent and common change and can be occasionally the only gross lesion granting a presumptive diagnosis of Johne's disease at necropsy. Advanced cases have signs of cachexia with loss of muscle mass, serous atrophy of fat deposits, intermandibular edema and effusions in body cavities. Plaques of intimal fibrosis and aortic mineralization have been reported. Focal granulomatous lesions can be occasionally found in liver, kidney and lungs.(4)
When gross lesions are well developed, the characteristic microscopic change, transmural granulomatous enteritis, is obvious. Characteristic histopathological finding in bovines is a transmural graulomatous enteritis, lymphadenitis and lymphangitis. Masses of epithelioid macrophages may accumulate in the submucosa. Foci of necrosis may occur within these aggregates of macrophages but in cattle, caseation and mineralization are extremely rare. Giant cells may be present. The inflammatory infiltrate may abnormally separate and displace crypts, which are elongate, with hyperplastic epithelium. Crypts may be distended with mucus and exfoliated cells. These lesions are characteristic of the multibacillary lepromatous phase of the disease as was in this case.(4,16) In cattle in which gross changes are minimal or absent, the microscopic abnormalities are more subtle. In these the lamina propria is diffusely infiltrated with lymphocytes and plasma cells, and a large number of eosinophils. There may be very few macrophages, and the most characteristic change is an infiltrate of lymphocytes and plasma cells in the submucosa, and associated with the submucosal and mesenteric lymphatics. Lymphangitis is one of the most consistent changes. Initially the lymphatics are surrounded by lymphocytes and plasma cells and many contain plugs of epithelioid cells in the lumen. Granulomas may form in the wall and project into the lumen. These nodules may undergo central necrosis. Granulomatous lymphadenitis occurs in mesenteric lymph nodes in advanced cases. In the early stages, there is histiocytosis of the subcapsular sinus. Ultimately, nodular or diffuse infiltrates of epithelioid macrophages and giant cells may replace much of the cortex, and infiltrate the medullary sinusoids.
In sheep and goats diarrhea is uncommon and the disease is characterized by chronic wasting. Enteric gross lesions are often mild, with little obvious thickening, and no transverse ridges; they are easily missed at necropsy. Gross lesions are similar but mineralized tubercle-like lesions have been reported.
Mycobacterium avium spp. paratuberculosis is an acid-fast, weakly Gram positive bacillus identifiable in the cytoplasm of macrophages as a rod shaped Ziehl-Neelsen positive organism. The organism is usually readily evidenced in macrophages and giant cells in the lesions when appropriately stained by acid-fast techniques. However, in some clinical cases, especially the ovine paucibacillary form, an extensive search may be needed for individual macrophages or giant cells bearing a few acid-fast bacilli. Johne's disease in sheep and especially in goats may resemble tuberculosis, on account of caseation and mineralization in granulomatous foci, and for such cases specific identification of the etiologic agent is necessary.
The antemortem diagnosis of Paratuberculosis in cattle is challenging and mirrors the evolution of immunopathological mechanisms elicited by MAPS. Serological tests such as ELISA and PCR are available, but bacteriological assay is still considered the gold standard diagnostic technique ((1); OIE http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/). Vaccination has been reported as an effective strategy for Paratuberculosis control in several countries.(1)
Mycobacterium avium spp. Paratuberculosis has been identified in intestinal tissue from patients with Crohn's disease by culturing the organisms and by PCR.(16) Additionally, some patients with Crohn's disease respond to antimycobacterial therapy.(3) The detection of genome or the isolation of MAPS could be caused by ingestion of contaminated food, but the hypothesis that multiple exposure to MAPS via alimentary route could be involved in the pathogenesis of Crohn's patients has been taken into account.(16)
JPC Diagnosis:
Conference Comment:
The type of granulomatous inflammation and clinical disease elicited by persistent, poorly degradable pathogens such as Mycobacterium species is dependent upon the hosts immune response.(16) Lepromatous inflammation is characterized by macrophages and epitheloid macrophages arranged in sheets within tissue. In tuberculoid lesions, the macrophages are arranged in distinct nodules (granulomas) that generally have a central necrotic core surrounded by a layer of macrophages, epithleoid macrophages and multinucleated giant macrophages, further surrounded by a layer of lymphocytes and plasma cells and bounded by fibroblasts and collagen.(16) The lepromatous form is thought to be associated with a strong Th2 (humoral) immune response, whereas the tuberculoid form is associated with a strong Th1 (cellular) immune response.(6) With a strong Th1 response, fewer Mycobacterium organisms are present; therefore this form is also referred to as the paucibacillary form. On the contrary, with a strong Th2 response, less effective intracellular bacterial killing results in more numerous intralesional bacteria; hence the lepromatous form of disease is also referred to as the multibacillary form.(6,16)
Recent studies of cytokines in red deer infected with M. avium subsp paratuberculosis suggest that Th2 and Treg immune responses may not play a direct role in clinical disease; but rather, may control the immunopathology of the disease and that it is the loss of these responses that leads to the development of clinical disease.(13)
References:
2. Begg DJ, Whittington RJ. Experimental animal infection models for Johne's disease, an infectious enteropathy caused by Mycobacterium avium subsp paratuberculosis. Veterinary Journal. 2008;176:129-145.
3. Borody TJ, Leis S, Warren EF, et al. Treatment of severe Crohn's disease using antimycobacterial triple therapy--approaching a cure? Dig Liver Dis. 2002;34:29-38.
4. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. 5th ed. Vol 2. Philadelphia, PA: Saunders Elsevier; 2007:69-128.
5. Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 1997;116:217-261.
6. Corpa JM, Garrido J, Garcia Marin JF, et al. Classification of lesions observed in natural cases of paratuberculosis in goats. J Comp Pathol. 2000;122:255-265.
7. Coussens PM. Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle. Infect Immun. 2004;72(6):3089-3096.
8. Dennis MM, Reddacliff LA, Whittington RJ. Longitudinal study of clinicopathological features of Johne's disease in sheep naturally exposed to Mycobacterium avium subspecies paratuberculosis.Vet Pathol. 2011;48:565-575.
9. Gonz+�-�lez J, Geijo MV, Garc+�-�a-Pariente C, et al. Histopathological classification of lesions associated with natural paratuberculosis infection in cattle. J Comp Pathol. 2005;133:184-196.
10. Greenstein RJ. Is Crohn's disease caused by a mycobacterium? Comparisons with leprosy, tuberculosis, and Johne's disease. Lancet Infect Dis. 2003;3:507-514.
11. Mann EA, Saeed SA. Gastrointestinal infection as a trigger for inflammatory bowel disease. Curr Opin Gastroenterol. 2012;28:24-29.
12. McKenna SL, Keefe GP, Tiwari A, et al. Johne's disease in Canada part II: disease impacts, risk factors, and control programs for dairy producers. Can Vet J. 2006;47(11):1089-1099.
13. Robinson MW, OBrien R, Mackintosh CG, et al. Immunoregulatory cytokines are associated with protection from immunopathology following Myocbacterium avium subspecies paratuberculosis infection in red deer. Infection and Immunity. 2011;79(5):2090-2097.
14. Scott PR, Clarke CJ, King TJ. Serum protein concentrations in clinical cases of ovine paratuberculosis (Johne's disease). Vet Rec. 1995;137:173.
15. Weiss DJ, Souza CD. Review paper: modulation of mononuclear phagocyte function by Mycobacterium avium subsp. paratuberculosis. Vet Pathol. 2008;45:829-841.
16. Zachary JF, McGavin DM. Pathologic Basis of Veterinary Disease. 5th ed. Philadelphia, PA: Elsevier Mosby; 2011;122-124, 172-173.