Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2011-2012
Conference 25
16 May 2012
Conference Moderator:
Donald Meuten, DVM, PhD, DACVP
Professor, Anatomic Pathology
North Carolina State University
College of Veterinary Medicine
Raleigh, NC 27607
CASE IV: N08-659 (JPC 3134301).
Signalment: 6-year-old castrated male bloodhound (Canis familiaris), canine.
History: Acute onset of renal failure. The dog died naturally.
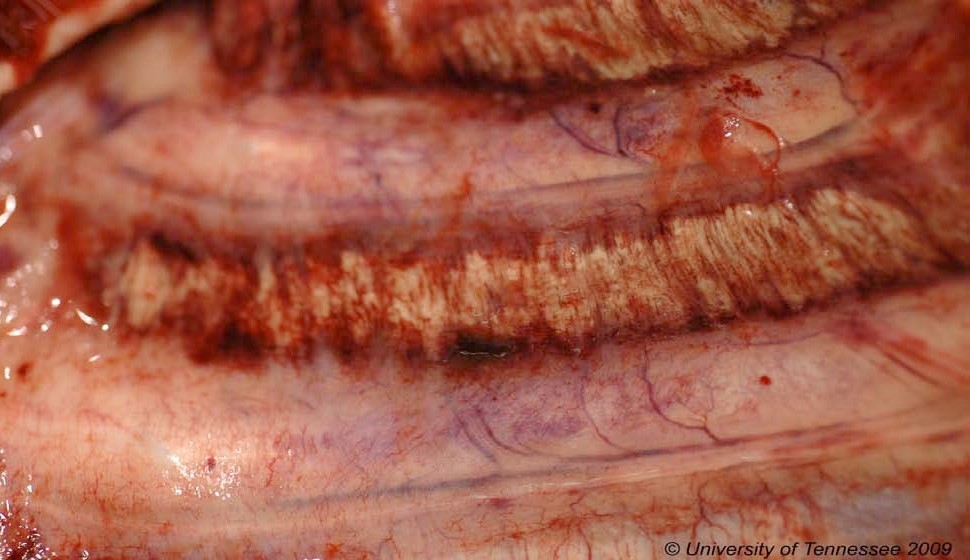
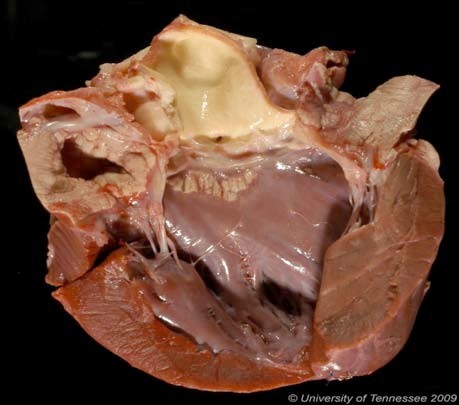
Gross Pathology: Both kidneys had a slightly granular (irregular) surface and were light brown. The parietal pleura and intercostal muscles were diffusely mineralized. The endocardial surface of the left atrium was diffusely mineralized; smaller areas of mineralization were present in the pulmonary artery. The lungs were mottled pink to purple, were irregularly firm. Approximately 50% of randomly sampled lung sections sank in formalin.
Laboratory Results: Plasma Chemistry: (only relevant findings listed)
BUN 155 mg/dL (9-24)
Creat 7.0 mg/dL (0.6-1.6)
Tot Prot 6.3 mg/dL (5.6-7.6)
Albumin 2.0 g/dL (3.1-4.2)
Globulin 4.3 g/dL (2.1-3.7)
A/G ratio 0.5 (0.9-1.9)
Calcium 8.9 mg/dL (9.9-11.5)
Phos 25.4 mg/dL (2.2-4.6)
Sodium 164 mEq/L (146-153)
Chloride 128 mEq/L (110-117)
Bicarb 12 mmol/L (16-23)
Anion Gap 28.6 (16-24)
Urinalysis: (only relevant findings listed)
Specific gravity 1.013
pH 7.0
Protein 2+
WBC 2-6
RBC 25-30
Rare transitional and squamous epithelial cells; Rare cellular casts
Urine Leptospirosis PCR â negative
Blood Gases
pH 7.051 (7.31-7.42)
Bicarb 13.6 (17-24)
Base excess -17.3 (0±2)
PO2 64.9 (85-95)
PCO2 48.6 (29-42)
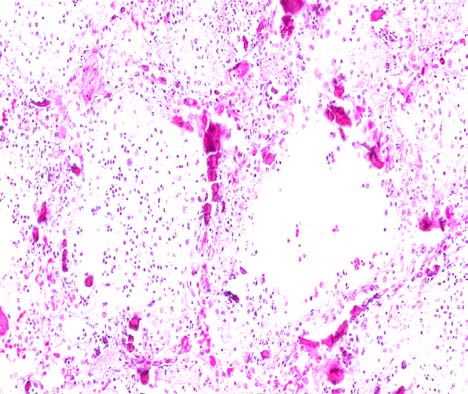
Contributor Histopathologic Description: Lung: There is widespread marked mineralization of bronchial walls and alveolar septa. The bronchial epithelium is occasionally ulcerated. The bronchial lumens and alveoli contain varying combinations and concentrations of fibrin, neutrophils, macrophages and erythrocytes. In some areas large, plump fibroblasts are present. Several blood vessels contain haphazardly arranged fibrin and neutrophils. Mutifocally there is fibrin, intermixed with neutrophils and macrophages, on the pleural surface.
In the left atrium (not submitted) there is marked mineralization surrounded by degenerate neutrophils, macrophages and karyorrhectic nuclear debris. The overlying endocardium is thickened. Similarly, the mesothelium lining the intercostal muscles (not submitted) is ulcerated and replaced by a sheet of fibrin. The underlying collagen is mineralized and surrounded by neutrophils, macrophages and karyorrhectic nuclear debris.
Contributor Morphologic Diagnosis: Lung: Marked acute diffuse mineralization and fibrinosuppurative pneumonitis.
Contributorâs Comment: The clinical presentation of uremic pneumonitis is consistent with adult respiratory distress syndrome (ARDS). Possible causes of ARDS include, but are not limited to: thermal or caustic injury, viral infections, ingested toxins, septicemia, disseminated intravascular coagulation, chronic left heart failure, pancreatitis, surfactant dysfunction, ventilator-induced injuries, adverse drug reactions, and uremia.2
Uremic pneumonitis results from diffuse alterations in alveolo-capillary permeability combined with complicating factors.1 These additional factors include, but are not limited to: alterations in coagulability1, fluid overload, pulmonary hypertension, decreased oncotic pressure, and metabolic acidosis-induced pulmonary vasoconstriction.5 The intensity of the azotemia does not correlate with the presence of uremic pneumonitis.1 One study examining the prevalence of uremic pneumonitis in humans that had died of glomerulonephritis found that uremic pneumonitis occurred exclusively in those patients that had rapidly progressive (and very inflammatory) mesangioproliferative glomerulonephritis.1
The uremia-associated damage to the alveolus and capillaries results in leakage of plasma into the alveoli and interstitium.1 The resultant histologic lesions are characterized by diffuse alveolar capillary damage, protein-rich interstitial and intra-alveolar edema, atelectasis, alveolar hemorrhages and hyaline membranes.5 Mineral is frequently deposited on the walls of the alveolar ducts and pulmonary arterioles.9 This tissue damage ultimately results in recruitment of neutrophils and macrophages. In rats, proteolytic enzymes and oxidants derived from the increased numbers of macrophages in the interstitial spaces are believed to be responsible for the destruction of elastic fibers, collagen and proteoglycans in the alveoli. Similarly, increased numbers of neutrophils mediate destruction through elastase, cathepsins, collagenase, myeloperoxidase and other oxidants.5 The resulting enzymatic and oxidative injuries exacerbate the uremia-associated damage to the alveolus and capillaries.
Cardiovascular lesions are also associated with uremia. Acute renal failure may result in fibrinoid vascular necrosis (arterial hyaline degeneration), while chronic renal failure produces hyperplastic arteriosclerosis. Mural endocarditis associated with uremia is typically confined to the left atrium and large elastic arteries, as was seen in this case. This lesion begins with deposition of glycosaminoglycans in the subendocardium/intima. This may progress to necrosis of the lining endothelium as well as the collagen, elastin and reticulin fibers with secondary inflammation. As in this case, concurrent mineralization can be marked.8
Nonrenal lesions of uremia are more likely to occur in cases of chronic renal failure than in cases of acute renal failure. Mineralization of the intercostal spaces, beneath the parietal pleura, as was seen in this case, is common. It is preceded by necrosis of the subpleural connective tissue with extension to the intercostal muscles and overlying parietal pleura. The pathogenesis of diffuse tissue mineralization in uremia is not completely clear. Uremic mineralization is likely a combination of dystrophic and metastatic mineralization. In dogs, renal failure is usually associated with hyperphosphatemia and hypocalcemia, as was seen in this dog. This may be complicated by concurrent renal secondary hyperparathyroidism.9
The increased anion gap in combination with decreased bicarbonate is consistent with a metabolic titration acidosis. The increased anion gap is likely due to the uremic acids. Additionally, the PCO2 is high (respiratory acidosis) and the PO2 is low (hypoxemia). The combination of these blood gas findings suggests decreased alveolar ventilation secondary to the marked uremic pneumonitis. This dog had both a metabolic and a respiratory acidosis.
Despite the acute onset of clinical signs of renal failure reported in this case, the renal lesions were chronic and consisted primarily of a lymphoplasmacytic interstitial nephritis with marked glomerulocystic change. The inciting cause of the renal failure in this dog is not known.
JPC Diagnosis: Lung: Alveolitis, chronic and necrotizing, focally extensive, severe, with marked mineratlization, hyaline membranes, emphysema, and fibrinous pleuritis.
Conference Comment: Even though there is hypocalcemia due to renal failure, there is still widespread soft tissue mineralization due to the drastic hyperphosphatemia. Mass law is the product of serum phosphorus and calcium, and soft tissue mineralization occurs when this exceeds 70.3 In the moderatorâs experience, the most common location for uremic pneumonitis is in the first third or fourth portion of the dorsal diaphragmatic lobes, and not in cranioventral lobes.
Conference participants discussed the fact that this dog likely had glomerular disease as the inciting cause of chronic renal failure because of the proteinuria and serum hypoalbuminemia. Renal and hepatic disease are the two primary sources of hypoalbuminemia, and only renal disease will preserve globulins as in this case. The two primary glomerular lesions in dogs in chronic renal failure are glomerulonephritis and amyloidosis. Cystitis is the most common cause of proteinuria in dogs, but concurrent hypoalbuminemia does not occur as in this case. The proteinuria must be evaluated in light of the erythrocytes present, which in this case are minimal, because hematuria contributes protein through albumin, globulin, and hemoglobin. Additionally, the urine dilution must be taken into consideration; the urine is dilute in this case, and therefore the proteinuria is significant.4,10,11,12
Conference participants differentiated this as a case of chronic renal failure from acute renal failure for the following reasons: the presence of serum hypoalbuminemia, since albumin has a half-life of around 20 days; the presence of glomerular disease, as acute renal failure of glomerular origin is extremely rare; the presence of soft tissue mineralization4,10,11,12, and the presence of non-regenerative anemia due to decreased erythropoietin production by the kidneys and uremia-induced erythrocyte fragility, since anemia is usually not present with acute renal failure.6
Conference participants discussed the azotemia as a mixture of pre-renal and renal origin, since the hypernatremia and hyperchloridemia point to dehydration and renal failure usually results in hyponatremia and hypochloridemia. Mixed metabolic and respiratory acidosis is present in this case, as mentioned by the contributor. In addition to the respiratory component being due to mineralization of pulmonary septa and decreased alveolar ventilation, pulmonary thrombosis is another likely cause, although this was not present in the slides.4,10,11,12 This is due to the loss of antithrombin III from glomeruli, placing the dog in a hypercoagulable state. The most common locations for thrombosis are the lungs and the aortic quadrification.7 Renal failure also usually produces vasculitis, especially in the midzonal region of vessels, and this is generally prominent in the lungs and gastrointestinal tract, although this was not present in this case.4,11
Contributor: University of Tennessee
College of Veterinary Medicine
Department of Pathobiology
2407 River Drive, Room A201
Knoxville, TN 37996-4542
References:
1. Bleyl U, Sander E, Schindler T. The pathology and biology of uremic pneumonitis. Intensive Care Med. 1981;7:193-202.
2. Caswell JL, Williams KJ. Respiratory system. In: Jubb, Kennedy, and Palmerâs Pathology of Domestic Animals. 5th ed. Vol. 2. Edinburgh, Scotland: Saunders Elsevier; 2007:564-567.
3. Cortadellas O, Fernandez del Palacio MJ, Talavera J, et al. Calcium and phosphorus homeostasis in dogs with spontaneous chronic kidney disease at different stages of severity. J Vet Intern Med. 2010;24:73-79.
4. Dibartola SP. Clinical approach and laboratory evaluation of renal disease. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 7th ed. Vol 2. St. Louis, MO: Saunders Elsevier; 2010:1955-69.
5. Heidland A, Heine H, Heidbreder E, et al. Uremic pneumonitis: evidence for participation of proteolytic enzymes. Contr Nephrol. 1984;41:352-366.
6. King LB, Giger U, Diserens D, et al. Anemia in chronic kidney failure. J Vet Intern Med. 1992;6(5):264-270.
7. Kuzi S, Segev G, Haruvi E, et al. Plasma antithrombin activity as a diagnostic and prognostic indicator in dogs: a retrospective study of 149 dogs. J Vet Intern Med. 2010;24(3):587-96.
8. Maxie MG, Robinson WF. Cardiovascular system. In: Jubb, Kennedy, and Palmerâs Pathology of Domestic Animals. 5th ed. Vol. 3. Edinburgh, Scotland: Saunders Elsevier; 2007:30.
9. Maxie MG, Newman SJ. Urinary system. In: Jubb, Kennedy, and Palmerâs Pathology of Domestic Animals. 5th ed. Vol. 2. Edinburgh, Scotland: Saunders Elsevier; 2007:432-436.
10. Polzin DJ. Chronic kidney disease. In: Bartges J, Polzin DJ, eds. Nephrology and urology of small animals. West Sussex, England: Wiley-Blackwell; 2011:433-471.
11. Polzin DJ. Chronic kidney disease. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 7th ed. Vol 2, St. Louis, MO: Saunders Elsevier; 2010:1990-2021.
12. Tripathi NK, Gregory CR, Latimer KS. Urinary system. In: Latimer KS, ed. Duncan and Prasseâs Veterinary Laboratory Medicine: Clinical Pathology. 5th ed. Ames, IA: Wiley-Blackwell; 2011:270-80.