CASE 2: MK18-01471 (4117513-00)
Signalment:
19-year-old, female, Rhesus macaque (Macaca mulatta)
History:
19-year-old female rhesus macaque with a history of anorexia, increased respiratory effort, swelling of the extremities and neck. Thoracic radiographs revealed an enlarged rounded heart which occupied the majority of the thoracic cavity. On auscultation, lung fields had wheezes and crackles. The heartbeat was faint. Due to the poor prognosis the macaque was euthanized.
Gross Pathology:
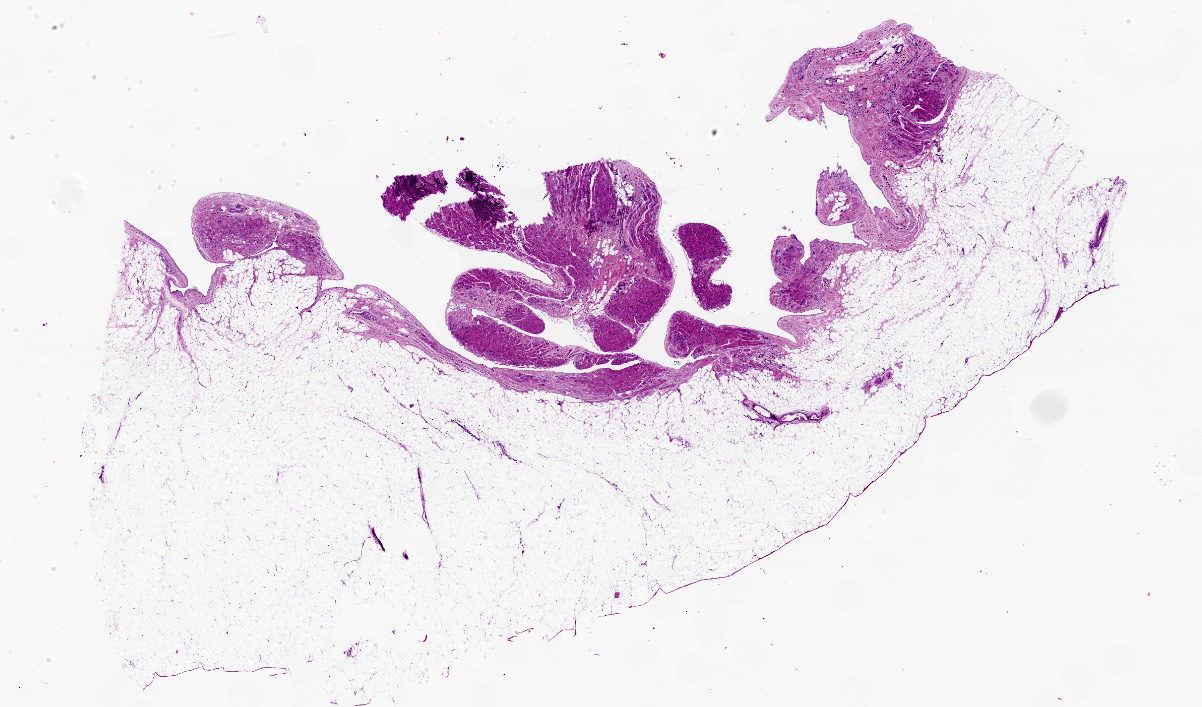
Examination revealed moderate subcutaneous edema involving the arms, legs and trunk. The macaque was obese with excessive body fat present. Approximately 200 ml of clear fluid was present in the abdomen with approximately 100 ml of similar fluid in the thorax. The heart was severely enlarged with severe dilatation of the right ventricle. The heart weighed 55 grams (0.04 % of body weight). There was excessive epicardial fat present, especially along the surface of the right ventricle. The wall of the right ventricle revealed large areas with little or no visible cardiac muscle. The left ventricle was small in profile compared to the extremely dilated right ventricle. The left ventricular free wall measured 0.6 cm, septum 0.5 cm, right ventricular free wall not including adjacent adipose tissue was 0.15 cm and with adjacent adipose tissue 0.45 cm. Sections of lung were atelectatic and had multifocal, up to 3-4 mm diameter, slightly raised gray to tan foci consistent with lung mite lesions. The liver was enlarged with rounded edges and was tan to brown with a lobular pattern, consistent with chronic passive congestion. A mass was present involving the body of the uterus which measures 3.5 x 3.5 x 4.0 cm. No other gross abnormalities were noted.
Laboratory results:
None.
Microscopic description:
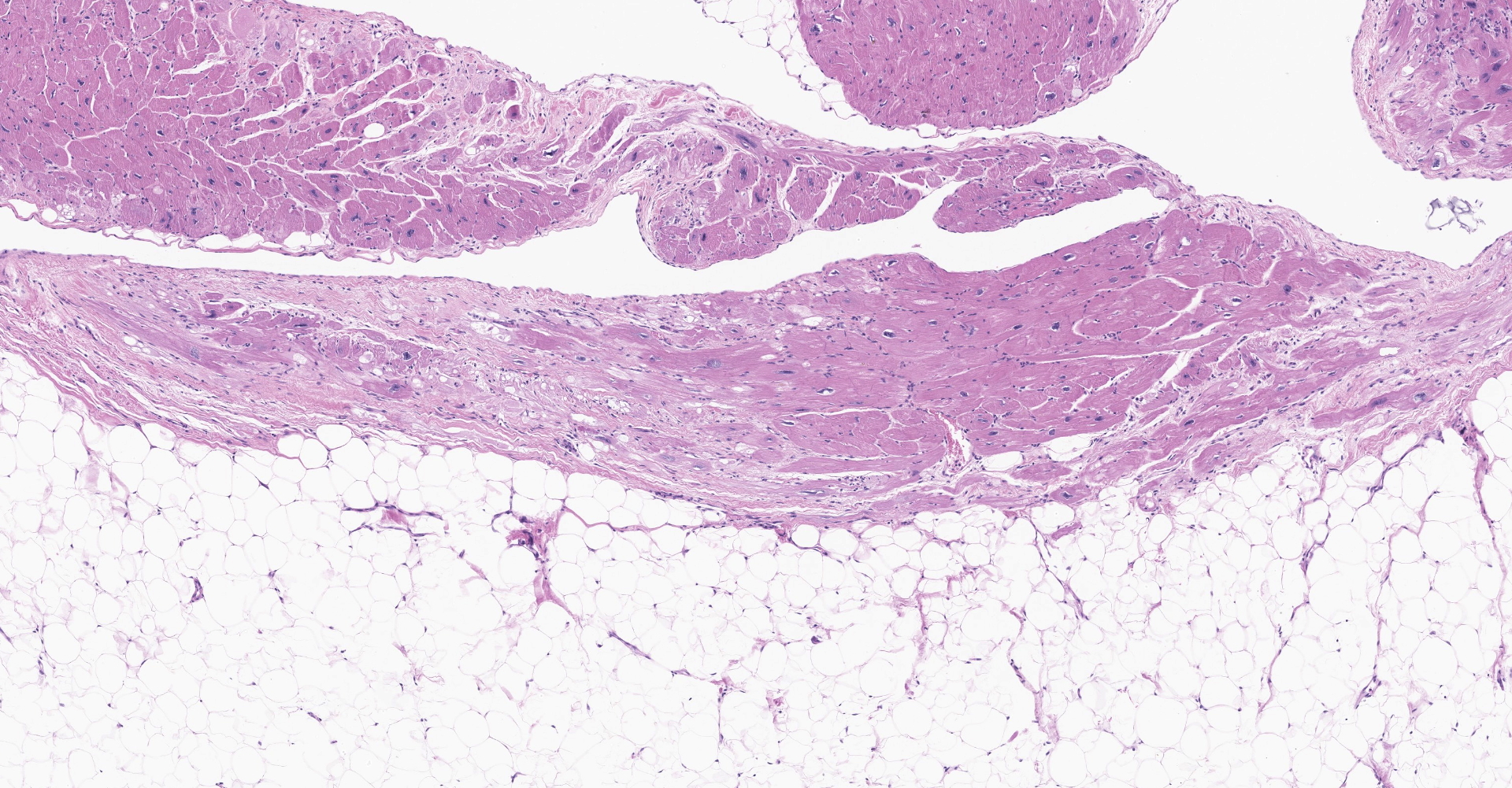
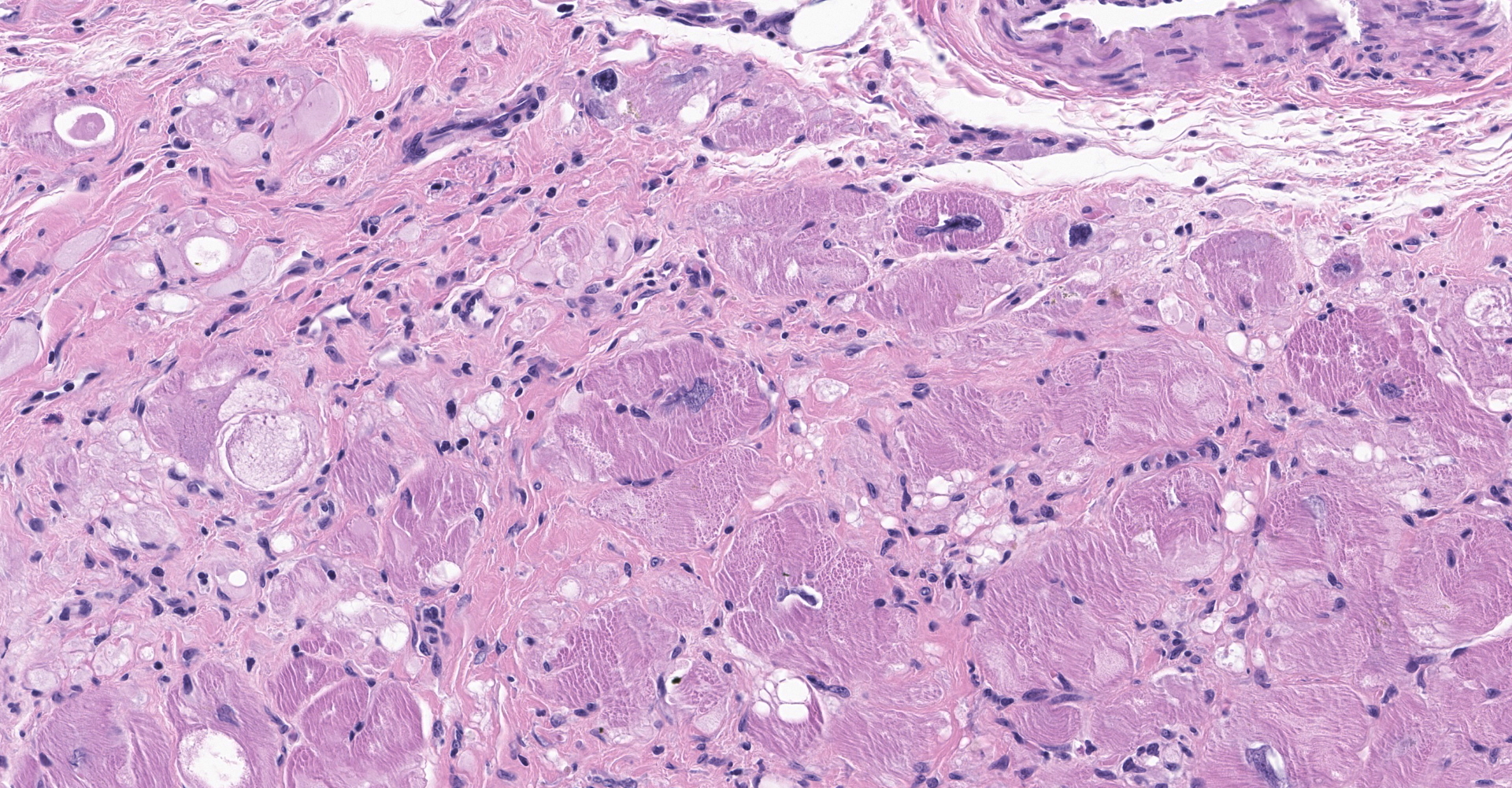
Heart: There is severe infiltration of adipose tissue within the wall of the right ventricle with severe atrophy and loss of myocytes. Remaining myocytes are hypertrophied with karyomegaly. Interstitial fibrosis is present adjacent to areas of existing myocytes and adjacent adipocytes. Focal mild to moderate fibrosis is noted in the interventricular septum. The left ventricle is unremarkable.
Other tissues: Liver: Scattered centrilobular regions are congested with pericentral necrosis evident (consistent with chronic passive congestion). There is moderate to severe periportal to midzonal lipidosis. Prominent pulmonary bronchiectasis with associated lung mites and peribronchiolar lymphoplasmacytic infiltrates were noted in the lung. The large uterine mass was a leiomyoma.
Contributor's morphologic diagnosis:
Rhesus macaque (Macaca mulatta) heart, cardiomyopathy, dilatative, right sided, severe with marked adipose deposition and myocyte atrophy. Fibrosis, interatrial septum and right ventricle and septum
Liver: Centrilobular necrosis and congestion
Liver: lipidosis, moderate to severe with centrilobular necrosis.
Contributor's comment:
Arrhythmogenic right ventricular cardiomyopathy (ARVC) was first reported in four male human patients in 1978 in France documenting clinical symptoms and EKG data. These patients experienced tachycardia with no prior clinical history, unusual right side QRS, S-T and conduction abnormalities. A subsequent report in 1982 included a study in which patients had right sided ventricular tachycardia along with decreased conduction by EKG. These patients presented with hypokinesis with localized dyskinesia. The histology revealed fatty infiltrates and fibrosis in cardiac tissue.11 Of twenty-two human athletes who died while participating in sports including soccer, long distance running, swimming and running, ARVC was found to be the cause of death in six cases.4
ARVC is a genetic disease linked to defects in 13 different genes that are vital components of the intercalated disc. In 50% of cases a familial genetic link can be identified leaving researchers to believe that other genes may be involved in this cardiomyopathy or that it may occur spontaneously. The first genetic link was reported in 1990 in which family history revealed a genetic mutation inherited at a higher ratio in males with variable penetrance and expression. This genetic autosomal dominant cardiomyopathy effects 1:2,000-5,000 people, typically RAeD diagnosed in 20-40-year-olds with predilection in males. In 50% of cases the most common symptom is sudden death while exercising.13
Most cases of ARVC are caused by functional mutations to ion channels, accessory proteins or structural genes. The intercalated disc has three components, the gap junction, the desmosome and the adheren junction, that function to keep the heart beating rhythmically. The gap junction is formed when connexins from two individual cells join making a channel. The connexins are made of six equal subunits who role is to move ions, sugar molecules, amino acids and nucleotides through the channel to synchronize the electrical potential of the two cells. A gene mutation of connexin 43 reduces the number of sodium channels subsequently creating a delay in repolarizing myocytes. This defect does not allow an adequate influx of Na+ leading to asynchronous beating of myocytes and eventual arrhythmias.15,18 The desmosome (macula adherens) provides structural support utilizing the intermediate filaments from neighboring cells. The desmosome has two structural parts, armadillo controlled by the JUP gene and plakins. Two proteins, desmoplakin and plakophilin exists in two adhesion states controlled by the amount of calcium. The plakins anchor the intermediate filaments. A defect found in ARVC to plakophilin 2 or desmoplakin weakens the bond with the intermediate filaments. The adherens junction provides strength, stability and adhesion between myocytes forming the bond between desmoglein and desmocollin in the intracellular space. It is formed by cadherins or laminins which form calcium dependent adhesions that also control cell fate, transduces signals and has other embryological roles. Defects in desmoglein 1, 2 and 4 and desmocollin 2 lead to weakening of the adherens junction and subsequent loss of function. It is this loss of function of the adherens junctions that cause remodeling of the intercalated disc, poor adhesion, apoptosis and fibrofatty replacement.5
The most common symptoms with ARVC are syncope, palpitations, chest pain and arrhythmias. Diagnosis is by unique EKG patterns that include repolarization and depolarization abnormalities and ventricular arrhythmias. Often the first sign of disease is sudden death. Definitive diagnosis is from family history, EKG and biopsy. Dilation is evident by ultrasound in the right ventricle. Histology reveals degenerating myocytes, some residual myocytes and replacement with adipose or fibrous tissue that may be transmural. Often trapped myocytes are seen along with an inflammatory response in the right ventricle with
thinning of the ventricular wall.16
Reports of ARVC have been documented in boxer dogs, a Siberian husky, a Weimaraner, cats and horses.6,7,9,10,12 The boxer dogs did not show signs of illness until the age of six with abrupt onset. As with humans, dogs that led the most sedentary lifestyle showed slower disease progression. The dogs that exercised most as with the human endurance athletes, were at greatest risk for sudden death. Genetic testing of the 49 dogs for striatin, which is instrumental in the formation of desmosomes revealed 33 had a gene mutation. Weakness to desmosomes can have both mechanical and electrical implications causing myocyte failure and arrhythmias due to poor conduction and cell to cell adhesion. The thinner wall of the right ventricle is unable to withstand the stress generated by exercise due to defects in cell to cell adhesion of myocytes.1
Two cats case histories documented ACRV in which both cats presented with pleural effusion, rapid breathing and an echocardiogram that revealed a very enlarged right ventricle and atrium. Histology of one cat heart revealed right ventricular dilation, fibrous and adipose replacement of myocytes, entrapped myocytes and inflammation. There was transmural fat replacement.10
Two horses' sudden death was investigated one occurring shortly after strenuous exercise. Grossly both hearts were gelatinous, and one had an area measuring 2 cm x 6 cm x 1 cm of marbled tissue. Histology showed a loss of myocytes with fatty tissue replacement and vacuolated Purkinje cells. There was collagenous tissue with stippled fibroblasts.9
Non-invasive treatments include restraint from strenuous exercise, use of beta blockers and anti-arrhythmic medications. Surgical options include placement of an implantable cardioverter defibrillator, catheter ablation of the affected heart tissue or heart transplantation.16
The mouse models have been generated including mutations for desmoplakin, plakophilin-2, desmoglein-2, desmocollin-2, plakoglobin and laminin receptor-1. Mouse models might provide insight if a gene mutation alters demise adhesion or is the loss of signaling pathways that are the first step in the it of myocytes. A JUP deficient mouse mimics the pathology experienced by endurance athletes. The mutant mice subjected to endurance training had evidence of ARVC four months earlier than their untrained cohorts. These mice had right ventricular dilation without the fibrofatty replacement.14
The findings in this case are consistent with right heart failure - ascites, pleural effusion, peripheral edema and chronic passive hepatic congestion. The right heart failure was due to significant loss of myocytes and infiltration of adipocytes and dilatation of the right ventricle. The appearance of the heart was similar to a rare condition in humans - AVRC.
Contributing Institution:
National Institutes of Health
Bethesda, MD 20892
https://www.ors.od.nih.gov/sr/dvr
JPC diagnosis:
Heart: Fibrofatty infiltration, focally extensive, severe, with cardiomyocyte degeneration, atrophy, and hypertrophy, karyomegaly, and endocardial and myocardial fibrosis.
JPC comment:
The contributor provides a concise summary of ARVC. A recent investigation into the death of an alpha male bonobo included genetic sequencing and analysis. There were no sufficiently variant sequences across 10 ARVC-associated genes (LMNA, CTNNA3, DES, TGFB3, JUP, TMEM43, PKP2, DSC2, DSG2, and DSP), but there were two variants of uncertain clinical significance in CTNNA3 and JUP that were not found in controls. It is currently not known whether these fully explain this bonobo's phenotype, but it may lead to additional research.2
Research into (human) diagnostics have recently focused on the identification of anti-cardiac desmoglein 2 (DSG2) autoantibodies. Desmoglein-2 is a cardiac cadherin protein that provides mechanical attachment between cells. Results showed that anti-DSG2 antibodies were specific to patients with ARVC, with no anti-DSG2 antibodies detected in controls without ARVC. Additionally, disease severity positively correlated with antibody level in these patients. Research into domestic species may find an equally suitable biomarker for this disease.3
A differential for ARVC in human literature is cardiac sarcoidosis, which is more typically characterized by granulomatous inflammation in the myocardium, often with giant cells and accompanied by lymphoplasmacytic inflammation. However, there are numerous presentations, depending on the progression of the disease at the time of diagnosis. Clinically, because there is disruption of Purkinje fibers, this condition may mimic ARVC and manifest with AV block, dysrhythmias, or sudden cardiac death. In cardiac sarcoidosis, granulomas form irregular, infiltrating yellow-tan, white-tan, or gray lesions and may involve any part of the heart. It has also been observed that when cardiac sarcoidosis presents as ARVC, endomyocardial biopsies are characterized by fibrofatty replacement without evidence of granulomatous inflammation.17
During conference discussion, it was noted between participants that there was some mild slide variation, with some sections having a mild endocarditis as well. The moderator emphasized the desmosome's role in intercalated disks and enumerated some of the documented genetic mutations in genes affecting components of desmosomes and intercalated disks in many cases of ARVC.
References:
1. Basso C, Fox PR, Meurs KM, et al. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation. 2004 Mar 9;109(9):1180-5
2. Celestino-Soper PBS, Lynnes TC Zhang L, et al. Genetic analyses in a bonobo (Pan paniscus) with arrhythmogenic right ventricular cardiomyopathy. Sci Rep. 2018;8(1):4350.
3. Chatterjee D, Fatah M, Akdis D, et al. An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. European Heart Journal. 2018;39:3932-3944.
4. Corrado D, Thiene G, Nava A, Rossi L, Pennelli N. Sudden death in young competitive athletes: clinicopathologic correlations in 22 cases. Am J Med. 1990 Nov;89(5):588-96.
5. Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ Res. 2010 Sep 17;107(6):700-14.
6. Eason BD, Leach SB, Kuroki K. Arrhythmogenic right ventricular cardiomyopathy in a weimaraner. Can Vet J. 2015 Oct;56(10):1035-9.
7. Fernandez del Palacio MJ, Bernal LJ, Bayon A, Bernabé A, Montes de Oca R, Seva J Arrhythmogenic right ventricular dysplasia/cardiomyopathy in a Siberian husky. J Small Anim Pract. 2001 Mar;42(3):137-42.
8. Frank R, Fontaine G, Vedel J, et al. Electrocardiologie de quatre cas de dysplasie ventriculaire droite arythmogene. Arch Mal Coeur 1978 71:963-72.
9. Freel KM, Morrison LR, Thompson H, Else RW. Arrhythmogenic right ventricular cardiomyopathy as a cause of unexpected cardiac death in two horses. Vet Rec. 2010 Jun 5;166(23):718-21.
10. Harvey AM, Battersby IA, Faena M, Fews D, Darke PG, Ferasin L. Arrhythmogenic right ventricular cardiomyopathy in two cats. J Small Anim Pract. 2005 Mar;46(3):151-6.
11. Marcus FI, Fontaine GH, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982 Feb;65(2):384-98.
12. Meurs KM, Stern JA, Reina-Doreste Y, Spier AW, Koplitz SL, Baumwart RD. Natural history of arrhythmogenic right ventricular cardiomyopathy in the boxer dog: a prospective study. J Vet Intern Med. 2014 Jul-Aug;28(4):1214-20.
13. Oomen AWGJ, Semsarian C Puranik R, Sy RW. Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy: Progress and Pitfalls. Heart Lung Circ. 2018 Apr 4. S1443-9506(18)30139-2.
14. Padron-Barthe L, Dominguez F, Garcia-Pavia P Lara-Pezzi E. Animal models of arrhythmogenic right ventricular cardiomyopathy: what have we learned and where do we go? Insight for therapeutics. Basic Res Cardiol. 2017 Sep;112(5):50
15. Paul M, Wichter T, Gerss J, et al. Connexin expression patterns in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2013 May 15;111(10):1488-95.
16. Rossi P Massumi A, Gillette P Hall RJ. Arrhythmogenic right ventricular dysplasia: clinical features, diagnostic techniques, and current management. Am Heart J. 1982 Mar;103(3):415-20.
17. Serei VD, Fyfe B. The many faces of cardiac sarcoidosis. Am J Clin Pathol. 2020;153(3):294-302.
18. Vila J, Pariaut R, Moise NS, et al. Structural and molecular pathology of the atrium in boxer arrhythmogenic right ventricular cardiomyopathy. J Vet Cardiol. 2017 Feb;19(1):57-67