Signalment:
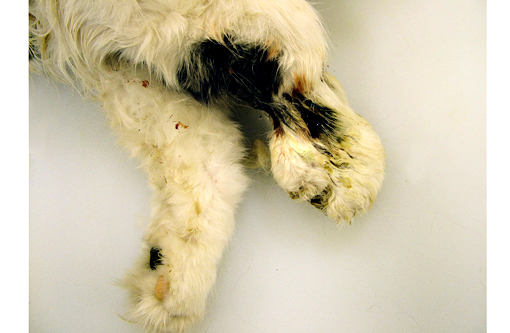
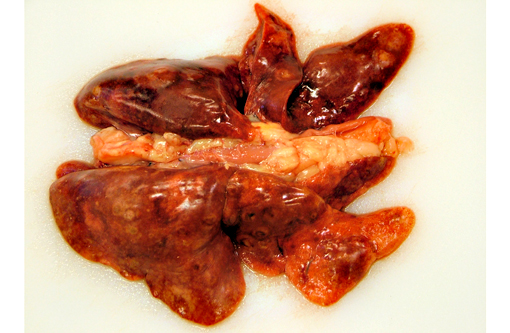
Gross Description:
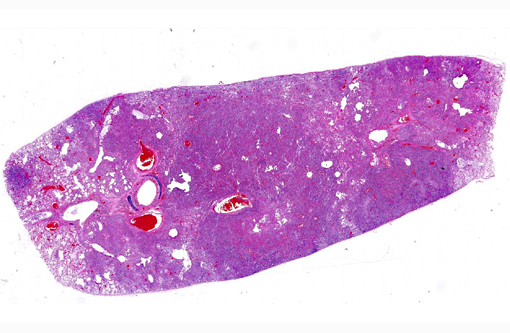
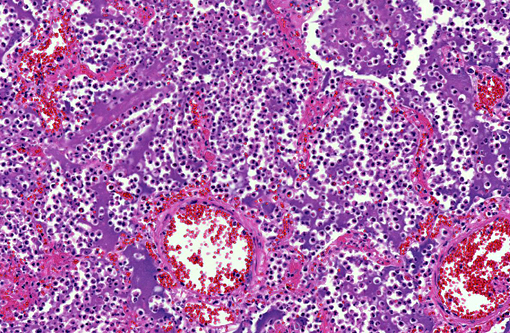
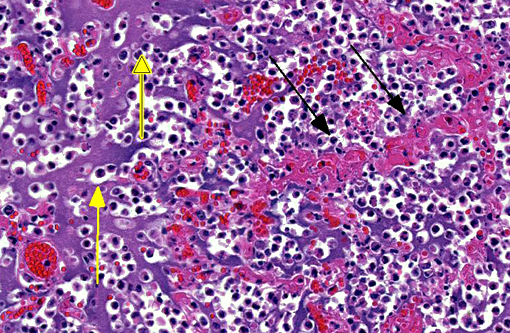
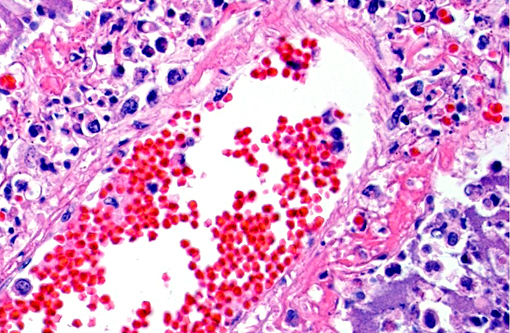
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Yersinia pestis is propagated in a flea-mammal (usually rodents) life cycle.(5,13) A flea capable of transmitting Y. pestis first must take a blood meal from a mammal that is septicemic with Y. pestis. The bacterium proliferates within the fleas proventriculus, eventually obstructing the fleas gastrointestinal tract. The gastrointestinal obstruction causes the flea to regurgitate Y. pestis into a mammalian host when the flea is taking subsequent blood meals. Although this is the accepted paradigm for flea transmission of plague, other methods of propagation have also been proposed.(6) Of the domestic species, cats are most susceptible to infection and disease, but dogs may also become infected and occasionally develop disease.(5,11) Cats are also more likely to transmit plague to people than dogs.(7,12) However, the cat flea, Ctenocephalides felis, is not an effective transmitter of Y. pestis.Â
Once Y. pestis is injected into a mammalian host by the flea, it expresses several virulence factors that help it to evade the mammalian host immune system.(5,9,10) Two virulence factors that help Y. pestis survive in the mammalian host are Yersinia outer coat proteins (YOPS) and a capsule consisting of fraction 1 protein (fraction 1 antigen). YOPS are injected into the hosts phagocytic cells via a type III secretory system to inhibit the inflammatory response by reorganizing the host cell cytoskeleton and inhibiting NF-κB suppressing cytokine production. YOPS can also be injected into endothelial cells, decreasing the expression of adhesion proteins on the cell surface. The capsule of Y. pestis is antiphagocytic and helps the bacterium to survive intracellularly when it is phagocytosed by a mammalian phagocyte, particularly monocytes and macrophages. The survival of Y. pestis within phagocytes helps it to be distributed from the initial site of infection to the regional lymph nodes and into the circulation.Â
The pathology of plague is similar in both cats and people.(5,10,13) The site of injection of Y. pestis at the flea bite can develop dermatitis and cellulitis, such as in this case, or more commonly has minimal inflammation. The most common presenting lesion is a swollen lymph node (bubonic plague) that may become necrotic and abscess. Y. pestis can then spread lymphogenously to other lymph nodes or hematogenously to other organs, particularly the organs that contain large numbers of resident macrophages (septicemic plague). In addition to the flea-mammal transmission, Y. pestis can be transmitted through the inhalation of aerolized Y. pestis (pneumonic plague) or through the ingestion of a Y. pestis infected rodent. Inhalation and ingestion of Y. pestis often results in a shorter incubation period of 1-3 days versus the 2-6 days incubation period after a flea bite due to the fact that the inhaled and ingested Y. pestis usually has already developed a capsule allowing the bacterium to spread more quickly in the host.(5)
JPC Diagnosis:
Conference Comment:
Plague is an endemic disease within the southwestern U.S., with prairie dogs being considered highly susceptible and important for disease transmission. A colony of Gunnisons prairie dogs has been identified as resistant to infection.(4) Only a minority of these animals mount an antibody response in the face of an outbreak, alluding to the role of the innate immune system as being responsible for conferring resistance to disease. Several proteins of the innate immune response, including VCAM-1, CXCL-1, and vWF, are upregulated in these animals following Yersinia exposure.(4)
Conference participants reviewed a vital component of the innate immune system: the pattern recognition receptors known as toll-like receptors (TLR). TLRs recognize pathogen-associated molecular patterns, or PAMPs, which are exogenous microbial products and include LPS, lipoteichoic acid, and peptidoglycans. This recognition of a microbe ultimately results in NF-κB activation which upregulates transcription of proteins important to the immune response. All TLRs except for two mediate NF-κB activation via MyD88 signaling, and thus are MyD88-dependent. TLR 4 has the ability to engage MyD88 or bypass it with TRAM and TRIF, resulting in type 1 IFN formation and release. TLR 3 operates exclusively through TRIF, and thus is MyD88-independent. TLRs can be characterized by which specific PAMPs they detect in addition to whether theyre located in the cell membrane or within the membrane of the endosome.(1) Understanding the specifics of TLR-signaling is often an important factor in elucidating disease pathogenesis and the corresponding immune response to a particular pathogen.Â
The contributor highlighted two important virulence factors of Yersinia spp., YOPS and fraction 1 antigen. YOPS are bacterial toxins which are injected into the cell. Three specific proteins (YopE, YopH, YopT) block phagocytosis by inactivating molecules that regulate actin polymerization. YopJ blocks inflammatory cytokine production by inhibiting LPS signaling pathways.(10)
References:
1. Ackermann MR. Inflammation and healing. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:111-113.
2. Ayyadurai S, Houhamdi L, Lepidi H, Nappez C, Raoult D, Drancourt M. Long-term persistence of virulent Yersinia pestis in soil. Microbiology. 2008;154:2865-2871.
3. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathologic Basis of Veterinary Disease. 5th ed. Vol. 2. Philadelphia, PA: Elsevier Saunders; 2007:204-205.
4. Busch JD, Van Andel R, Stone NE, et al. The innate immune response may be important for surviving plague in wild Gunnisons prairie dogs. J Wildl Dis. 2013;49(4):920-931.
5. Chomel BB. Plague. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier; 2012:469-476.
6. Eisen RJ, Bearden SW, Wilder AP, et al. Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. PNAS. 2006;103:15380-5.
7. Gage KL, Dennis DT, Orloski K A, et al. Cases of cat-associated plague in the western US, 1977-1998. Clin Infec Dis 2000;30:893-900.
8. Hirsh DC. Enterobacteriaceae: Yersinia. In: Hirsh DC, MacLachlan NJ, Walker RL, eds. Veterinary Microbiology. 2nd ed. Ames, IA: Blackwell; 2004:75-80.
9. Li B, Yang R. Interaction between Yersinia pestis and the host immune system. Infect Immun. 2005;76(5):1804-1811.
10. McAdam AJ, Sharpe AH. Infectious diseases. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders: 2010:331-398
11. Nichols MC, Ettestad PJ, VinHatton ES, et al. Yersinia pestis infection in dogs: 62 cases (2003-2011). J Am Vet Med Assoc. 2014;244(10):1176-1180.
12. Pennisi MG, Egberink H, Hartmann K, et al. Yersinia pestis infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2013;15:582-584.
13. Perry RD, Fetherston JD. Yersinia pestis etiologic agent of plague. Clin Microbiol Rev. 1997;10(1):35-66.