Signalment:
Gross Description:
Histopathologic Description:
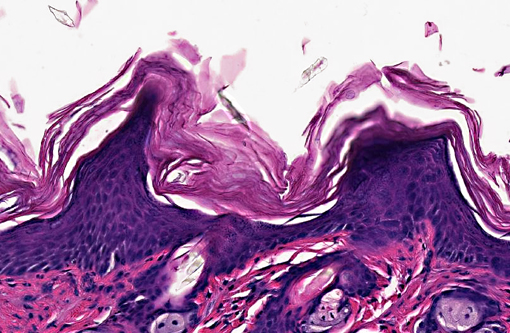
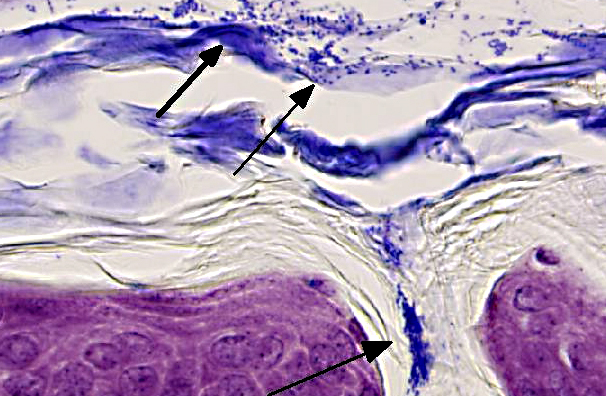
Skin (from Dam B): Sparsely haired, non-pigmented skin. Diffusely, there is marked orthokeratotic hyperkeratosis, and multifocal small, ~10 μm, -Ç-ÿdusty intracorneal bacterial colonies within follicles and in superficial keratin. The bacteria are very small basophilic coccobacilli. Multifocally, there is mild but conspicuous thickening of the stratum spinosum up to 5 cell layers (acanthosis), and the superficial dermis contains low numbers of lymphocytoid mononuclear cells.
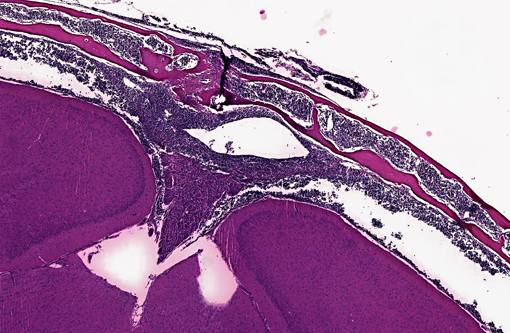
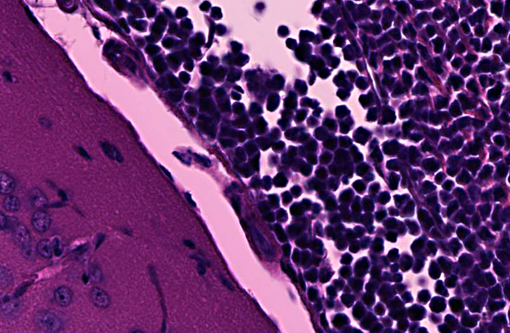
Transverse section of head, decalcified (from Dam A): Expanding the bone marrow and spilling out into the adjacent subdural space and meninges, and ventrally surrounding the pituitary and cranial nerves, is an unencapsulated, poorly delineated, neoplastic cellular infiltrate consisting of a monomorphic population of round cells (lymphocytes) multifocally coalescing into densely cellular sheets. Cytomorphology is somewhat compromised by demineralization of the head specimen; however, neoplastic lymphocytes are characterized by distinct cell borders, a moderate amount of eosinophilic granular cytoplasm, a round to oval centrally located nucleus, stippled chromatin, and occasionally 1-2 indistinct nucleoli. Mitoses are 0-1/HPF, and anisocytosis and anisokaryosis are minimal. There is multifocal minimal hemorrhage, and mild individual cell necrosis (pyknotic nuclei, and karyorrhectic debris).
Morphologic Diagnosis:
1. Skin, dam: Hyperkeratosis, orthokeratotic, diffuse, marked with mild acanthosis, minimal non-suppurative dermatitis, and intracorneal bacterial colonies
2. Head, dam, bone marrow, meninges, subdural space: Lymphoma, leukemia.
Lab Results:
| Dam A | Dam B | |
| WBC | (K/μl) 4.44 | 22.8 |
| NE (K/μl) | 2.64 | 12.8 |
| Ly (K/μl) | 0.99 | 4.11 |
| Eo (K/μl) | 0.25 | 1.79 |
| Ba (K/μl) | 0.02 | 0.17 |
| Mo (K/μl) | 0.54 | 3.92 |
| RBC (M/μl) | 7.91 | 10.44 |
| PLT (K/μl) | 800 | 440 |
Condition:
Contributor Comment:
In the current case, histopathology of pups combined with PCR results confirmed infection with Corynebacterium bovis in this NSG colony. The section on this slide is from the worst affected of the 2 dams. Corynebacterium bovis, the agent of scaly skin disease in mice, is a commensal and opportunistic bacterial agent that causes hyperkeratosis, pup mortality, and failure to thrive in susceptible mouse strains including immunodeficient nude, NOD SCID, and NSG mice.(1,2,3,7) Morbidity can be high, as transmission and persistence in the environment are associated with shedding of the infected keratin flakes.(8) Mortality is generally low, except in young/suckling mice.(8) Some immunocompetent mice may develop skin disease, but often harbor the bacteria without clinical signs, and thus serve as a reservoir, making it difficult to eradicate. Additionally, bacteria can persist in the environment, and can spread through sloughed keratin debris to other cage mates, or iatrogenically through handlers, which serve as a vehicle for transmission.(1,8) In addition to C. bovis infection, histologic evaluation of the gastrointestinal tract revealed a diverse enteric flora, including protozoa, corroborating the conventional microbial status of these animals. These findings strongly suggest that severely immunodeficient NSG mice require more stringent barrier conditions and microbial exclusion in order to thrive and be useful for research purposes.(10) The very mild dermatitis here was characterized as non suppurative, instead of lymphocytic or lymphoplasmacytic, because mice this genotype are expected not to have lymphocytes.
Interestingly, both submitted dams from this small group of breeding NSG mice had disseminated lymphoma with marrow and vascular involvement (leukemia). The head section is from Dam A. Cytomorphology, which is easier to assess in non-decalcified tissues, was consistent with a lymphoblastic lymphoma. Immunohistochemistry (on Dam A) was positive for CD3 and CD43, and negative for B220 and PAX5. These findings are consistent with T cell lymphoblastic lymphoma.(6) A similar presentation of lymphoma has been reported in the NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, mouse, sometimes called NOG, which is also on a NOD.scid background but has a different loss of the function mutation in Il2rg.(5) These findings represent the first documented reports of lymphoma in NSG mice, which have been considered to be completely lymphocyte deficient, resistant to lymphocyte -Ç-ÿleakiness and resistant to lymphoma.(7) Immune stimulation associated with Corynebacterium bovis and other agents encountered with less stringent barrier conditions may predispose to lymphoma, and may compromise the research value and lifespan of immunodeficient mice.
JPC Diagnosis:
1. Haired skin: Hyperkeratosis, orthokeratotic, diffuse, moderate, with moderate epidermal hyperplasia and intracorneal bacilli.
2. Head, at level of hippocampus, cranial bone marrow, meninges, and tissues ventral to brain: Lymphocytic leukemia.
Conference Comment:
The use of various stains of scid mice in biomedical research originated from four littermates of the C.B-17 inbred strain that were found to lack IgM, IgG1, and IgG2a during a serum immunoglobulin quantitation study in 1983. These mice lacked both T and B lymphocytes because of a heritable recessive mutation, similar to a condition reported in humans.(9) Scid mice have a spontaneous loss-of-function mutation of the protein kinase, DNA activated, catalytic polypeptide (Prkdc) gene. Prkdc plays a role in repairing double-stranded DNA as well as in recombining the variable (V), diversity (D) and joining (J) segments of immunoglobulin and T cell receptor genes. Because of their inability to complete V(D)J gene recombination, T and B cells cannot mature; thus affected animals cannot develop cell mediated or humoral immune responses.
The penetrance of the scid mutation varies, and reduced penetrance can result in animals that are leaky, developing immunoglobulin and T and B cells as they age.(3) Approximately 15% of scid mice have detectable serum Ig and functional T cells, and 15% of aged C.B17/Icr-scid/scid mice and 67% of 40-week-old NOD scid/scid mice develop thymic T-cell lymphomas.(9) Thus, as the contributor states, NSG mice, which are generally resistant to such leakiness are becoming increasingly popular in research. Nonetheless, the contributor provides a compelling argument that exposure to opportunistic pathogens may both circumvent the strains natural resistance to leakiness, and contribute to the development of lymphoma; this case, therefore, underscores the importance of maintaining strict barrier conditions and microbial exclusion to preserve the research value of immunodeficient mice.
The relatively non-specific JPC histologic diagnosis of lymphocytic leukemia is based on the evaluation of hematoxylin- and eosin-stained sections available to conference participants. We believe that this diagnosis is attainable by conference participants; a more specific diagnosis of T-cell lymphoblastic leukemia would be appropriate if participants had access to non-decalcified sections and immunohistochemical studies performed by the contributor.
References:
2. Foreman O, Kavirayani AK, Griffey SM, Reader R, Shultz LD. Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Vet Pathol. 2011;48:495-499.
Jackson Laboratory Mouse Strain Data. Scid mice overview. http://jaxmice.jax.org/support/straindata/scid.html. Accessed 2 March 2013.
3. Jacoby RO, Fox JG, Davidson M. Biology and diseases of mice. In: Fox JG, Anderson LC, Loew FM, Quimby FW, eds. Laboratory Animal Medicine. 2nd ed. San Diego, CA; Elsevier Science. 2002:92-93.
4. Kato C, Fujii E, Chen YJ, Endaya BB, Matsubara K, Suzuki M, et al. Spontaneous thymic lymphomas in the non-obese diabetic/Shi-scid, IL-2R γnull mouse. Laboratory Animals. 2009;43:402-404.
5. Morse HC, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, et al. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood. 2002;100:246-258.
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ. The Jackson Laboratory. Bar Harbor, ME. http://jaxmice.jax.org/literature/factsheet/SFS0001_005557.pdf, 2010
6. Scanziani E, Gobbi A, Crippa L, Giusti AM, Pesenti E, Cavalletti E, et al. Hyperkeratosisassociated coryneform infection in severe combined immunodeficient mice. Laboratory Animals. 1998;32:330-336.
7. Sudenberg JP, Shultz LD. The Severe Combined Immunodeficiency (scid) Mutation. JAX-« Notes. 1993: 453. http://jaxmice.jax.org/jaxnotes/archive/453a.html. Accessed online on 2 March 2013.
8. Treuting PM, Clifford CB, Sellers RS, Brayton CF. Of mice and microflora: considerations for genetically engineered mice. Veterinary Pathology. 2012;49:44-63.