Signalment:
Gross Description:
Histopathologic Description:
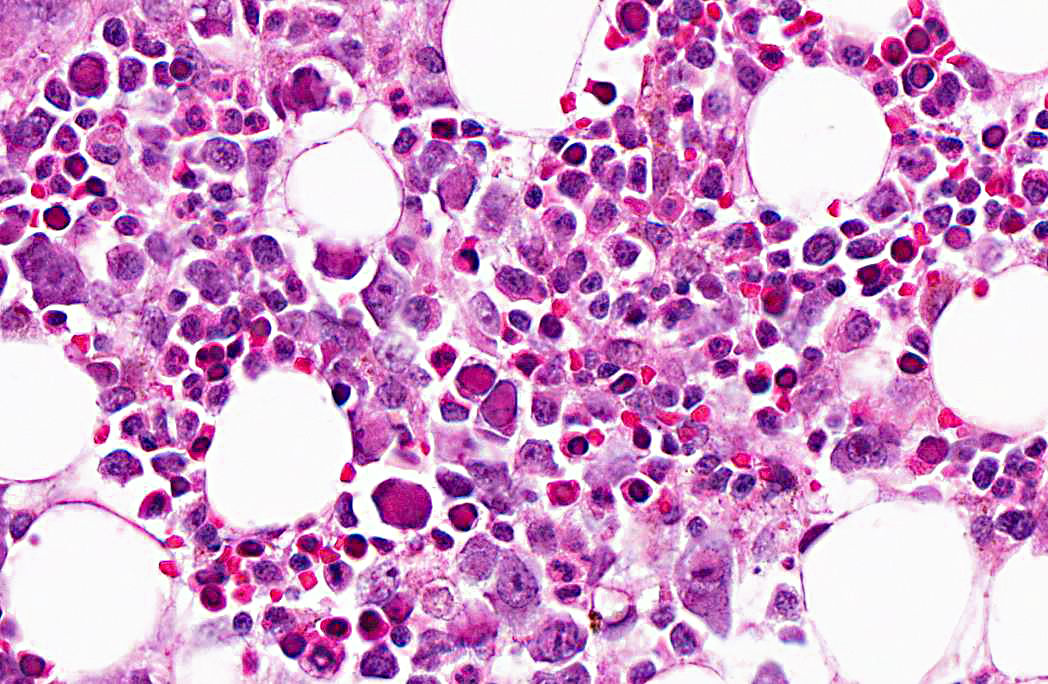
Microscopic examination of femoral bone marrow revealed hypercellular marrow with abnormal erythroid cells with bizarre nuclear forms and intranuclear inclusions. These cells have a glassy intranuclear eosinophilic inclusion body, stained pink or lilac, that displaces the chromatin to the periphery.
Ultrastructural examination revealed intranuclear viral particles and an occasional viral array characteristic of parvoviruses.
Morphologic Diagnosis:
Lab Results:
| Hematology Parameters | Pre-study | Post renal transplant Day 28 | Post whole blood transfusion Prior to necropsy |
| RBC - μl | 5.31 | 2.31 | 2.93 |
| Hemoglobin - gm% | 12.8 | 5.1 | 6.9 |
| Hematocrit (HCT) - % | 37.6 | 14.9 | 19.0 |
| Reticulocyte count (RETIC) - % RBC | 0.0 | 0.3 | 2.7 |
| Mean corpuscular volume (MCV) - fl | 70.8 | 64.5 | 64.8 |
| Mean corpuscular hemoglobin (MCH) - pg | 24.1 | 22.1 | 23.5 |
| Mean corpuscular hemoglobin concentration (MCHC) - g/dL | 34.0 | 34.2 | 36.3 |
| Platelets | 377000 | 373000 | 619000 |
| WBC | 8700 | 2860 | 1420 |
| Neutrophils | 2436 (28%) | 1801 (63%) | 908 (64%) |
| Lymphocytes | 6003 (69%) | 858 (30%) | 454 (32%) |
| Monocytes | 87 (1%) | 143 (5%) | 56 (4%) |
| Eosinophils | 174 ( 2%) | 57 (2%) | 0 (0%) |
Condition:
Contributor Comment:
In humans, B19 can persist at low levels in the bone marrow of infected humans for extended periods, establishing latency, and a similar situation can be anticipated to occur in macaques with the related SPV. Both viruses target rapidly dividing cells and demonstrate a tropism for cells within the erythrocytic lineage. In immunologically normal animals, infection has not been associated with clinical disease, but with immunosuppression or immunodeficiency; infection may cause anemia and widespread infection of erythroid cells. In bone marrow, poorly-defined eosinophilic intranuclear inclusions may be observed, in association with dyserythropoiesis.(4) Ultrastructural examination and in situ hybridization can be used to confirm the diagnosis.(4) Such infections and pathology have been observed in both SIV- and SRV-infected rhesus macaques, as well as in immunosuppressed cynomolgus macaques, in which severe clinical anemia has been diagnosed.(1,4)
A SPV infection can be particularly problematic in transplantation studies in which NHPs received some form of immunosuppressive therapy to prevent transplant rejection. Using a combination of molecular detection techniques such as PCR and serologic testing, monkeys can be screened prior to initiation of studies and the selection of viral-negative animals will help prevent transmission from donor to recipient. However, immunosuppression allows latent infection to manifest itself, as in this case.
JPC Diagnosis:
1. Bone marrow: Erythrocytic hyperplasia, mild to moderate.
2. Bone marrow, erythrocytic precursors: Intranuclear viral inclusions, numerous.
Conference Comment:
The family Parvoviridae is divided into two subfamilies, Parvovirinae and Densovirinae. Parvovirinae contains viruses of vertebrates, and Densovirinae viruses affect insects. The Parvovirinae subfamily is further divided into five genera: Parvovirus, Erythrovirus, Dependovirus, Amdovirus, and Bocavirus.(3) The following tables list parvoviruses of veterinary importance and the diseases associated with them:(3)
Genus Parvovirus
| Virus | Disease |
| Feline Panleukopenia Virus | Generalized disease in kittens; panleukopenia; enteritis; in-utero or neonatal infection can cause cerebellar hypoplasia; raccoon, mink and coatimundi are also susceptible |
| Mink Enteritis Virus | Leukopenia; enteritis |
| Canine Parvovirus 2 (3 major variants: subtypes 2a, 2b, 2c) | Generalized disease in puppies; enteritis; myocarditis; lymphopenia |
| Porcine Parvovirus | Stillbirth, mummification, embryonic death, infertility (SMEDI); rare respiratory disease, vesicular disease and systemic disease of neonates |
| Rodent Parvoviruses (Mouse parvoviruses, minute virus of mice, Kilhams rat virus, Toolanss H-1 virus of rats) | Subclinical or persistent infection; fetal malformations; hemorrhagic syndrome in rats; Confounding effects on research; hamsters with hamster parvovirus develop periodontal and craniofacial deformities |
| Rabbit Parvoviruses (Lapine Parvovirus) | Usually no clinical signs; may produce disseminated infection, mild enteritis in young kits |
Genus Erythrovirus
| Virus | Disease |
| Non-Human Primate Parvoviruses (Simian Parvovirus, Rhesus Parvovirus, Cynomolgus Parvovirus) | Anemia; fetal abnormalities; elatedto human B19 virus |
Genus Amdovirus
| Virus | Disease |
| Aleutian Mink Disease Virus | Adults (primarily mink that are homozygous for Aleutian coat color, which is associated with a Chediak- Higashi type abnormality): Chronic immune complex disease; encephalopathy; Neonates: interstitial pneumonia |
Genus Dependovirus
| Virus | Disease |
| Goose Parvovirus | Hepatitis; myocarditis; myositis; lethal disease in 8-30 day old goslings |
| Duck Parvovirus | Hepatitis; myocarditis; myositis in Muscovy ducks |
Genus Bocavirus
| Virus | Disease |
| Bovine Parvovirus | Rarely associated with clinical disease; mild diarrhea in neonates |
| Canine Parvovirus 1 (Canine Minute Parvovirus) | Subclinical; rarely causes diarrhea or sudden death in neonates |
References:
2. OSullivan MG, Anderson DK, Goodrich JA, Tulli H, Green SW, Young NS, et al. Experimental infection of cynomolgus monkeys with simian parvovirus. J Virol. 1997;71:45174521.
3. Parris CR. Parvoviridae. In: Maclachlan JN, Dubovi EJ, eds. Fenner's Veterinary Virology. 4th ed. New York, NY: Academic Press; 2011:225-235.
4. Simon MA. Simian parvoviruses: biology and implications for research. Comp Med. 2008;58:47-50.