CASE 3: O240/19.6 (4152972-00)
Signalment:
Species: Dog (Canis familiaris)
Breed: Mixed breed (Pointer x Labrador)
Gender: Male, castrated
Age: 4 years old
History:
The dog presented with depression, acute-onset ataxia with mild bilateral forelimb hypermetria and mild pain on palpation of the thoracic spine. Rectal temperature was 39.1 °C and C-reactive protein (CRP) 14.3 mg/L (reference range 0-10 mg/L). The dog was treated with steroids, antimicrobials and sedation, but during the next 24 hours it deteriorated to non-ambulatory recumbency with seizures. The dog was subsequently euthanized and submitted for necropsy.
Gross Pathology:
Two approximately 0.5 cm in diameter areas of light red discoloration was present on the surface of the cerebral frontal lobes, one in each hemisphere. Meningeal blood vessels were prominently dilated and the brain tissue diffusely slightly edematous.
Laboratory results:
RT-qPCR of tissue from the brain: positive for tick-borne encephalitis virus (TBEV)
Microscopic description:
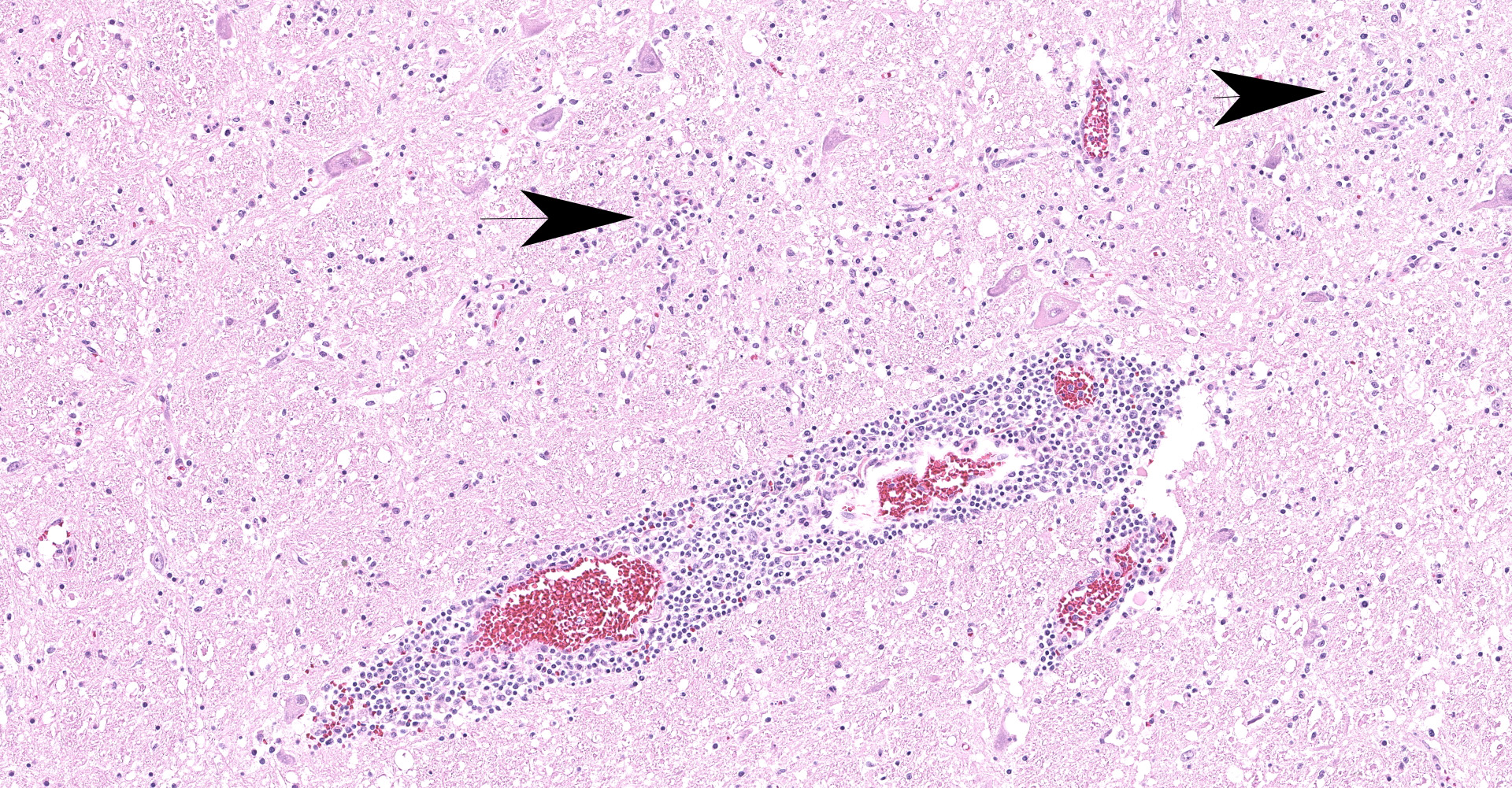
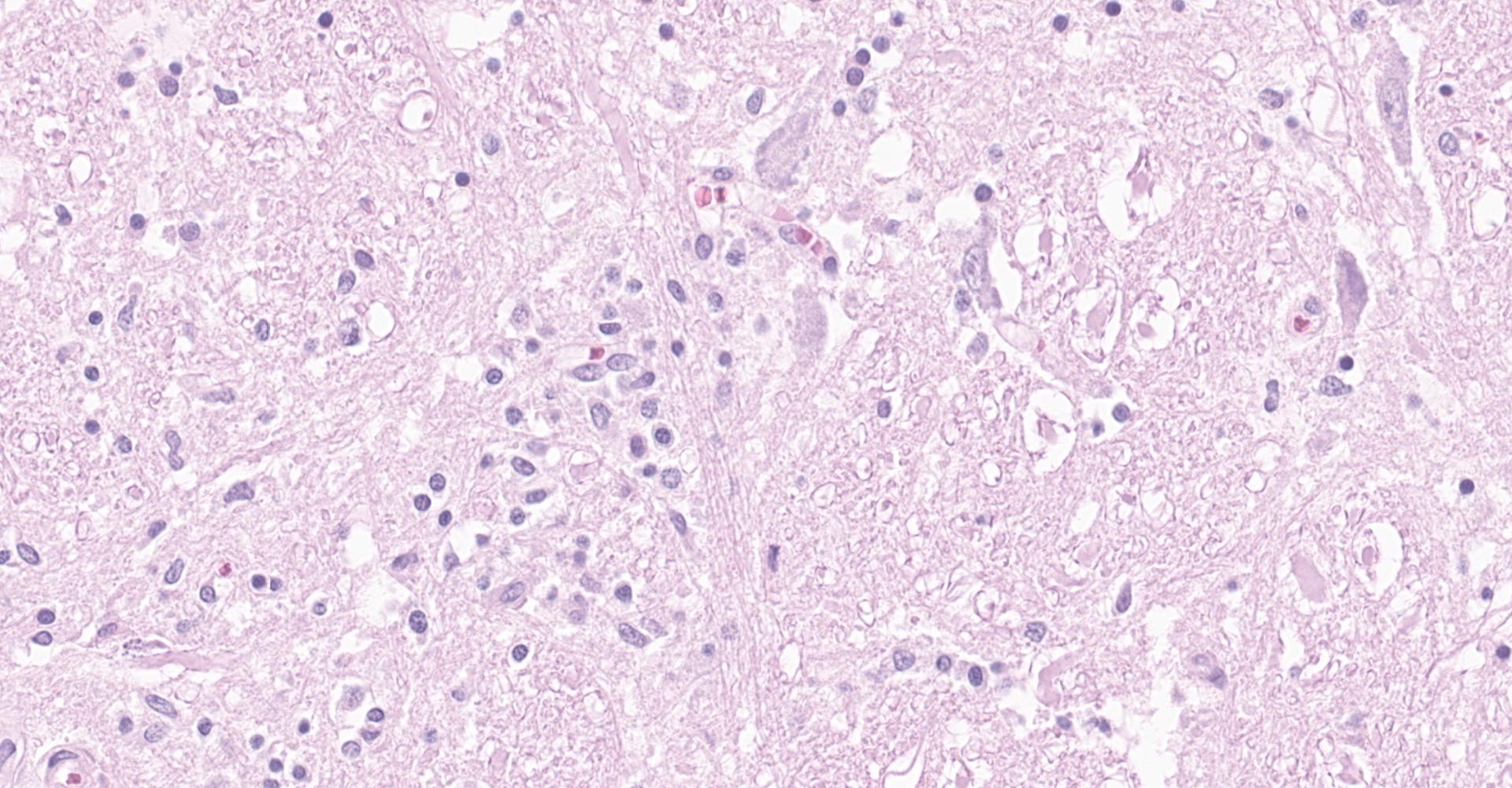
Brainstem, level of medulla oblongata. Both white and grey matter show multifocal to coalescing severe inflammatory infiltrates comprising lymphocytes, plasma cells and histiocytes, located within the neuroparenchyma and surrounding vessels as up to 10 layer thick perivascular cuffs. Affected vessels are outlined by hypertrophic endothelium, often blood filled and occasionally show mild separation by clear spaces between vessel walls and surrounding parenchyma (perivascular edema). Multifocally, there is mild to moderate vacuolation of the neuroparenchyma (rarefaction). The neuroparenchyma shows multifocal nodular to diffuse gliosis, as well as satellitosis and neuronal degeneration and loss. Multifocally, cells display hypereosinophilic shrunken cytoplasm with pycnotic or karryorhectic nuclei (necrosis). Occasionally, microglia are seen surrounding neuronal debris (neuronophagia). In addition, multifocal to coalescent infiltrates comprising moderate numbers of lymphocytes and plasma cells, and occasional histiocytes are present within the leptomeningeal subarachnoid space and surround leptomeningeal vessels as perivascular cuffs.
Contributor's morphologic diagnosis:
Meningoencephalitis, lymphoplasmacytic and histiocytic, necrotizing, multifocal to coalescing, severe, with gliosis and glial nodule formation
Contributor's comment:
Tick-borne encephalitis virus (TBEV), belonging to the genus flavivirus and family Flaviviridae5, is the causative agent of the zoonotic disease tick-borne encephalitis (TBE).5,13 TBEV is a positive-sense, single-stranded RNA (+ssRNA) enveloped virus with a viral genome of approximately 11kb in length.5,10 There are three subtypes of TBEV; the Western European subtype, the Siberian subtype and the Far Eastern subtype.5,13 The tick vector for the European subtype is Ixodes ricinus, whereas Ixodes persulcatus is the main vector for the Siberian and Far Eastern subtypes.5,10 Small mammals, mainly rodents, serve as virus reservoirs and play a major role in the spread of TBEV.7,13
TBEV is of major health concern in humans, with thousands of human cases reported annually in endemic areas of Europe and Asia.1,5 Humans are typically infected after being bitten by ticks, but in approximately 1% of human cases, the source of infection is unpasteurized dairy milk and milk products containing TBEV.10 Several wild and domestic animals are also susceptible to TBEV1, including sheep, dogs and horses.13 In contrast to humans, TBEV infection in these species commonly causes seroconversion, but rarely causes clinical disease.1,4,7,13 Only a few clinical cases of TBEV infection in dogs have been previously reported in Europe.7,14 However, the clinical cases seen in dogs present as very severe, with a high fatality rate.1,7 In acute cases, death commonly occurs within a week.4
After a bite from a tick infested with TBEV, the virus replicates in Langerhans cells and neutrophils in the hosts' skin, upon which dendritic cells migrate to the site of infection. Dendritic cells are subsequently activated and bring the virus to regional lymph nodes.10,13 After viral replication in the lymph nodes, spread into the bloodstream and primary viremia occurs. The virus is then able to infect various organs, especially spleen, liver and thymus, thereby causing a secondary viremia.10,13 In this stage of the disease, the infection may be cleared, and seroconversion may occur without development of clinical signs.10,13 In patients with high viral titers or insufficient neutralizing TBEV specific antibodies, TBEV may however infect the brain during the secondary viremia by crossing the blood brain barrier (BBB).10,13
The exact mechanism behind the viral crossing of BBB is not known, but probably includes a combination of several proposed ways; infection of microvascular endothelial cells of the BBB, cytokine/chemokine mediated BBB disruption, direct axonal retrograde transport from infected peripheral neurons into somatic motor neurons in the spinal cord, infection of olfactory neurons or transportation of virus by infected immune cells that traffic to the CNS.10 Inside the brain, the virus replicates in large neurons of the anterior horns, medulla oblongata, pons, dentate nucleus, Purkinje cells and striatum.10
Both humoral and cellular immunity seem to be required to clear TBEV infection from a vertebrate host. Antibody responses include TBEV-specific IgM and IgG antibodies in serum and cerebrospinal fluid and are critically important in controlling and clearing the infection, primarily by targeting the E and NS1 protein of TBEV.10 Both neutralizing antibodies and in part non-neutralizing antibodies play a role in prevention of the disease. Neutralization occurs by several mechanisms, including direct neutralization of receptor binding, post-binding or pre-fusion neutralization inside endosomes, and Fc receptor-mediated clearance by cells of the reticuloendothelial system.10
TBEV-infected neurons in the CNS are also targets for cytotoxic T-cells (CD8+). Whether or not T-cell responses are protective or pathological during TBE seems to depend on several factors, for example the pathogenicity of a particular TBEV strain, infectious dose and immunological status of host.9,10 In transfer of CD8+ T-cells into SCID mice infected with TBEV, mean survival time was decreased, meanwhile after infection with low-pathogenic TBEV strain, CD8+ T-cells seemed to increase survival.9 Possibly, CD4+ T-cells also prevent lethal TBE through CD4+-mediated secretion of IFN-? and other pro-inflammatory cytokines, and/or stimulation of macrophage-like cells.9
The incubation period in dogs is estimated to be between 5-9 days.4 The clinical signs reflect multifocal neurological lesions in the cerebrum and brain stem, including elevated body temperature and behavioral changes such as depression, altered consciousness, proprioceptive deficits, hyporeflexia in front and/or hind legs, paresis, generalized ataxia, tetraplegia, tremor, convulsions, cervical hyperalgesia, vestibular syndrome, and cranial and/or spinal nerve function deficits.1,4,7
The histopathological changes seen in TBEV infection include necrotizing lymphoplasmacytic and histiocytic meningoencephalomyelitis with severe neuronal necrosis and neuronophagia, diffuse gliosis and glial nodule formation, and non-purulent perivascular cuffing in both grey and white matter.1,14 Almost the entire brain may be affected but the most severe changes are typically seen in basal ganglia, thalamus, mesencephalon, neuroparenchyma surrounding fourth ventricle and medulla oblongata.1,7,14 Focal microgliosis (so called "glial shrubbery") in the molecular layer of cerebellum has been reported.14
A possible histopathological differential diagnosis to TBE in dogs comprise encephalitis caused by another flavivirus; the West Nile (WN) virus. Equids are most susceptible to WN disease, but subclinical disease may occur in canids. The histologic lesions in WN infection resemble those caused by TBEV, but in WN neuronal degeneration and necrosis is not as severe, and lesions are more confined to the grey matter. In addition, glial nodules may contain a few neutrophils. Furthermore, in WN infection lesions are overall often milder and restricted to the brain stem and thoracolumbar spinal cord, where axonal swelling and spheroid formation may be seen.1
Confirmation of TBEV infection include RT-PCR analysis of serum or cerebrospinal fluid7,12, or post-mortem detection of viral antigen in brain tissue by RT-PCR or immunohistochemistry.7,14 Serum IgM or IgG TBEV-specific antibody titers may be measured by immunofluorescence assays (IFA) or ELISA, but possible cross reaction with other flaviviral antibodies (such as WN virus-specific antibodies) may interfere with the results.2,3,7 Under such circumstances, specific immunity against TBE virus may be assessed in virus neutralization assays.2
Contributing Institution:
Swedish University of Agricultural Sciences
Department of Biomedical Sciences and Veterinary Public Health, Section of Pathology
BOX 7028
SE 750 07, Uppsala, Sweden
https://www.slu.se/en/departments/biomedical-sciences-veterinary-public-health/
JPC diagnosis:
Brainstem: Meningoencephalitis, lymphohistiocytic, diffuse, moderate, with astrogliosis, glial nodules, and rare spheroids.
JPC comment:
The contributor provides an excellent review of this Flaviviral disease in dogs. As noted above this virus is of more paramount concern in human populations, can affect some domestic species, but does not often cause clinical disease. However, canine cases have reported pyrexia, changes in behavior and mentation, and apathy. Affected animals have exhibited forelimb or hindlimb paresis, quadriplegia, seizures, convulsions, ataxia, proprioceptive deficits, neck hyperesthesia, facial nerve paralysis, strabismus, anisocoria, nystagmus, miosis, loss of corneal reflex, and optic neuritis. Using seroprevalence studies, canine infection rates may be as high as 11.6% in endemic areas, but clinical manifestations are seen in rare instances. In endemic areas of Austria and German, seroprevalence rates in horses is as high as 26.1% and 20-30%, respectively, with only rarely reported cases of clinical disease. Infections in ruminants are largely asymptomatic, but experimental infection has been demonstrated in lambs. A small number of captive exotic species have been reportedly infected, including a macaque (Macaca sylvanus), a markhor (Capra falconeri) and a reindeer (Rangifer tarandus).11
Infection by ingestion of milk represents an interesting transmission mechanism. Human infection through this method often results in family clusters, as opposed to the less organized infection patterns through ticks. TBEV is sensitive to its environment, but milk allows it to survive exposure to acidic gastric contents for up to two hours. Within that timeframe, most of the ingested contents are found in the lower gastrointestinal tract, allowing for infection by the intact virus through M cells in Peyer's patches.11
In human TBEV infections, rare cases of hepatic compromise have been reported. One study found that 22.2% of patients had abnormal liver function tests. A separate study found approximately 22% of patients had elevated liver enzymes, with the most often affected values in AST and ALT. While most cases of TBEV in animals present with advanced neurologic symptoms, in animals presenting with non-specific signs, a complete blood chemistry panel may help identify an etiology.6
Because humans are more severely affected by TBEV than dogs, and humans and dogs often live in close proximity, dogs are a potential sentinel species. Studies in Scandinavian dogs illustrated geographic areas where TBE was more common than originally expected, new foci of TBEV, and showed the need for better and more extensive TBEV surveillance. In Belgium, a serologic screening of 880 dogs revealed only two ELISA-positive cases, and 8 borderline positive cases. While Belgium does not consider this disease to be endemic, this provides a baseline for subsequent seroprevalence studies which might inform future policy, screening, and treatment.8
References:
- Cantile C, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 6th ed. Philadelphia, PA:Saunders Elsevier; 2016:392-394.
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21:36-40.
- Klaus C, Ziegler U, Kalthoff D, Hoffman B, Beer M. Tick-borne encephalitis virus (TBEV) ? findings on cross reactivity and longevity of TBEV antibodies in animal sera. BMC Vet Res. 2014;10:78.
- Leschnik MW, Kirtz GC, Thalhammer JG. Tick-borne encephalitis (TBE) in dogs. Int J Med Microbiol. 2002;291:66-69.
- Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371:1861-71.
- Mrzljak A, Tabain I, Premac H, et al. The Role of Emerging and Neglected Viruses in the Etiology of Hepatitis. Current Infectious Disease Reports. 2019;21:51.
- Pfeffer M, Dobler G. Tick-borne encephalitis virus in dogs ? is this an issue? Parasit Vectors. 2011;4:59.
- Roelandt S, Heyman P, De Filette M, et al. Tick-Borne Encephalitis Virus Seropositive Dog Detected in Belgium: Screening of the Canine Population as Sentinels for Public Health. Vector-Borne and Zoonotic Diseases. 2011;11(10):1371-1376.
- Rusek D, Salát J, Palus M, et al. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology. 2008;384(1):1-6.
- Ruzek D, Av?i? ?upanc T, Borde J, et al. Tick-borne encephalitis in Europe and Russia: review of pathogenesis, clinical features, therapy and vaccines. Antiviral res. 2019;164:23-51.
- Salat J, Ruzek D. Tick-borne encephalitis in domestic animals. Acta virologica. 2020;64(2):226-232.
- Schwaiger M, Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J Clin Virol. 2003;27:136-145.
- Valarcher JF, Hägglund S, Juremalm M, et al. Tick-borne encephalitis. Rev Sci Tech OIE. 2015;34(2):453-66.
- Weissenböck H, Suchy A, Holzmann H. Tick-borne encephalitis in dogs: neuropathological findings and distribution of antigen. Acta Neuropathol. 1998;95:361-366.