Signalment:
Two-year-old, male, spayed female, beagle (
Canis familiaris).The dog
presented for chylothorax after lobectomy at a referral surgical center several
months before. After surgery, there was a persistent effusion that was managed
with an indwelling Pleura-Port. The dog received a short course of cyclosporine
for the effusion. On recheck, there was a significant decline in clinical
condition (not really consistent with a cylothorax). Radiographs at that time
showed the development of a diffuse milliary pattern not reported on original
films or seen at rDVM a few weeks earlier. Based on the report, cyclosporine
was discontinued and the dog was sent to the rDVM (owner's relative) to
consider options. The plan was for the patient to return for lung biopsy in a
few days but the dog continued to decline and after a night on oxygen with
minimal stabilization
and much less improvement, euthanasia was performed. Only lung tissue was
submitted by the clinician for histopathology.
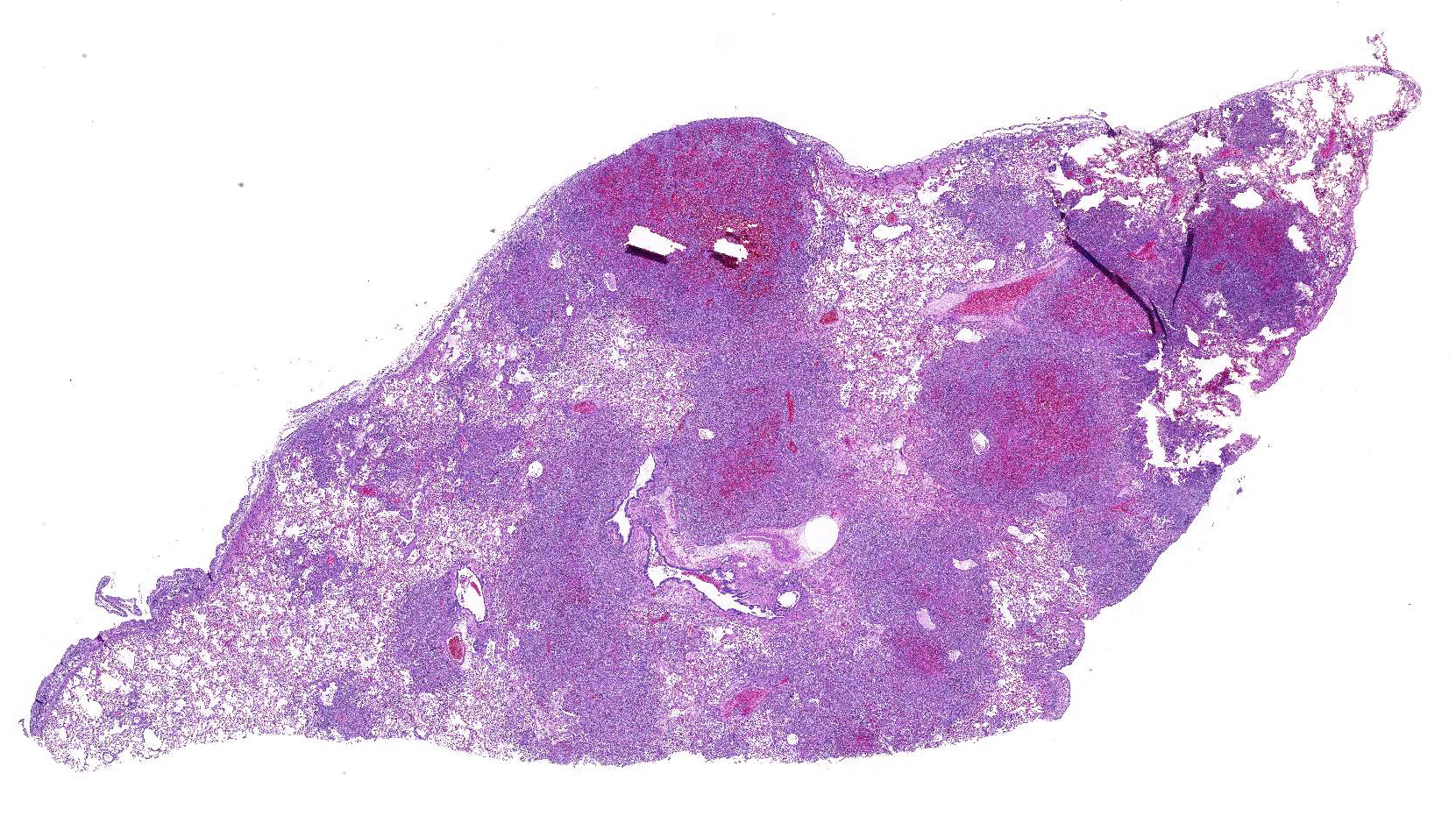
Gross Description:
Sections
of formalin-fixed lung were mottled tan-red on cut section.
Histopathologic Description:
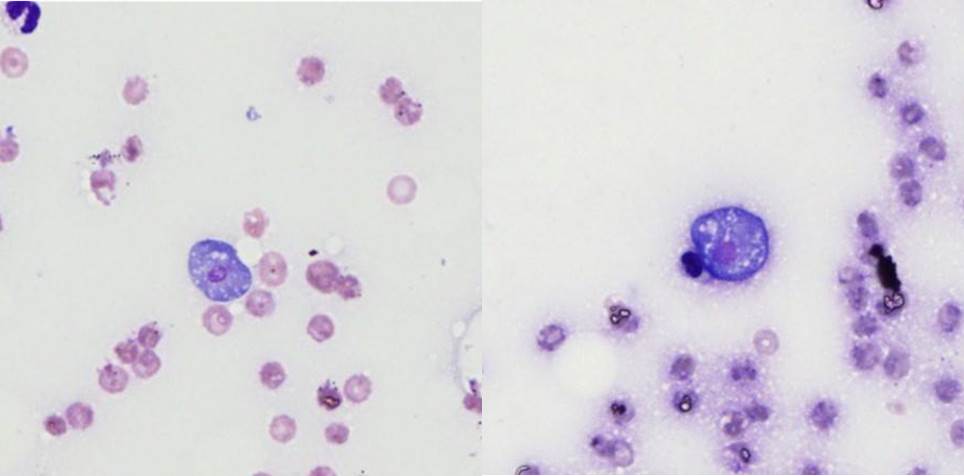
Lung:
There are extensive multifocal to coalescing areas of tissue necrosis admixed
with hemorrhage, fibrin, edema, karyorrhectic and occasionally mineralized
debris; these areas are variably centered on partially or fully effaced
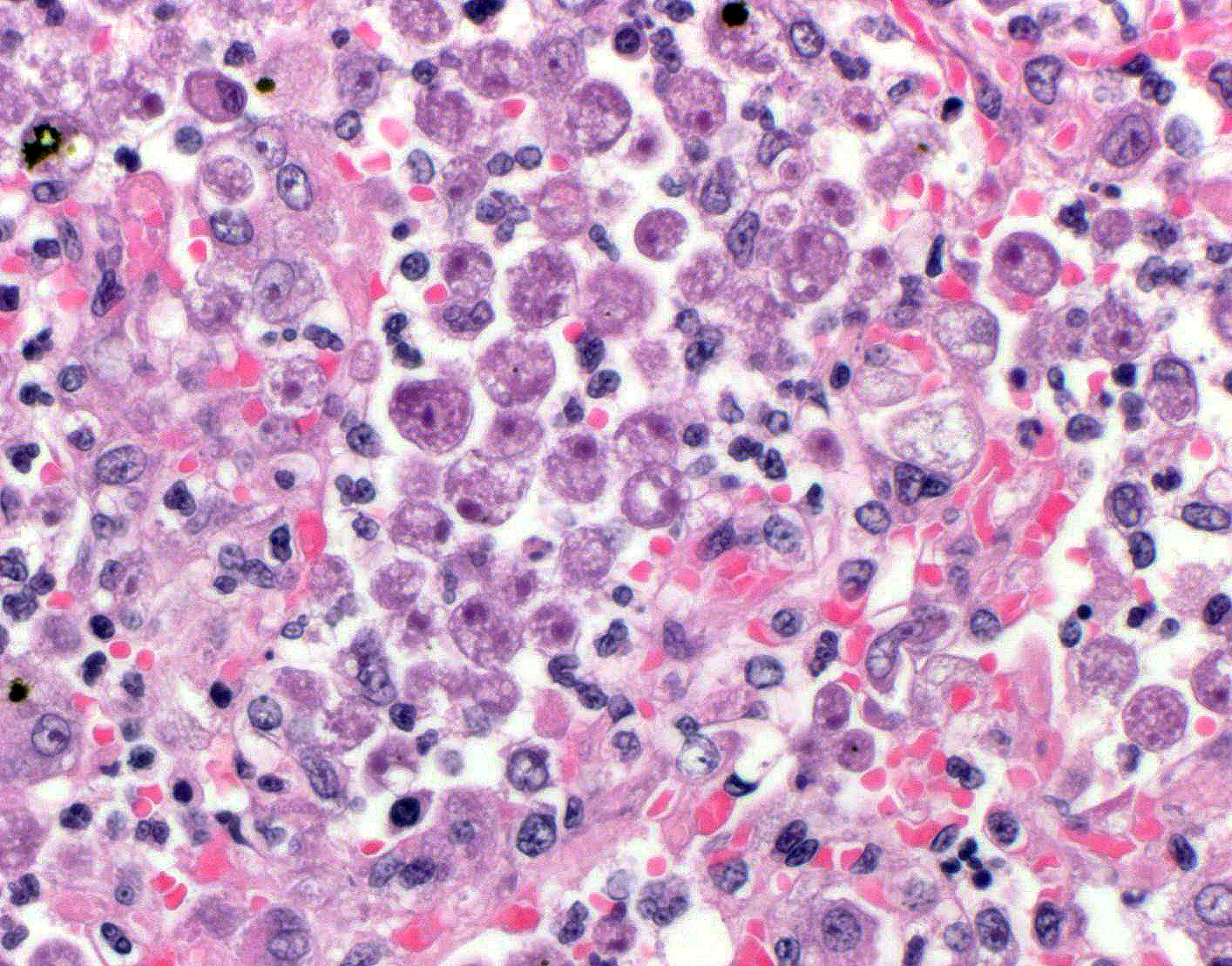
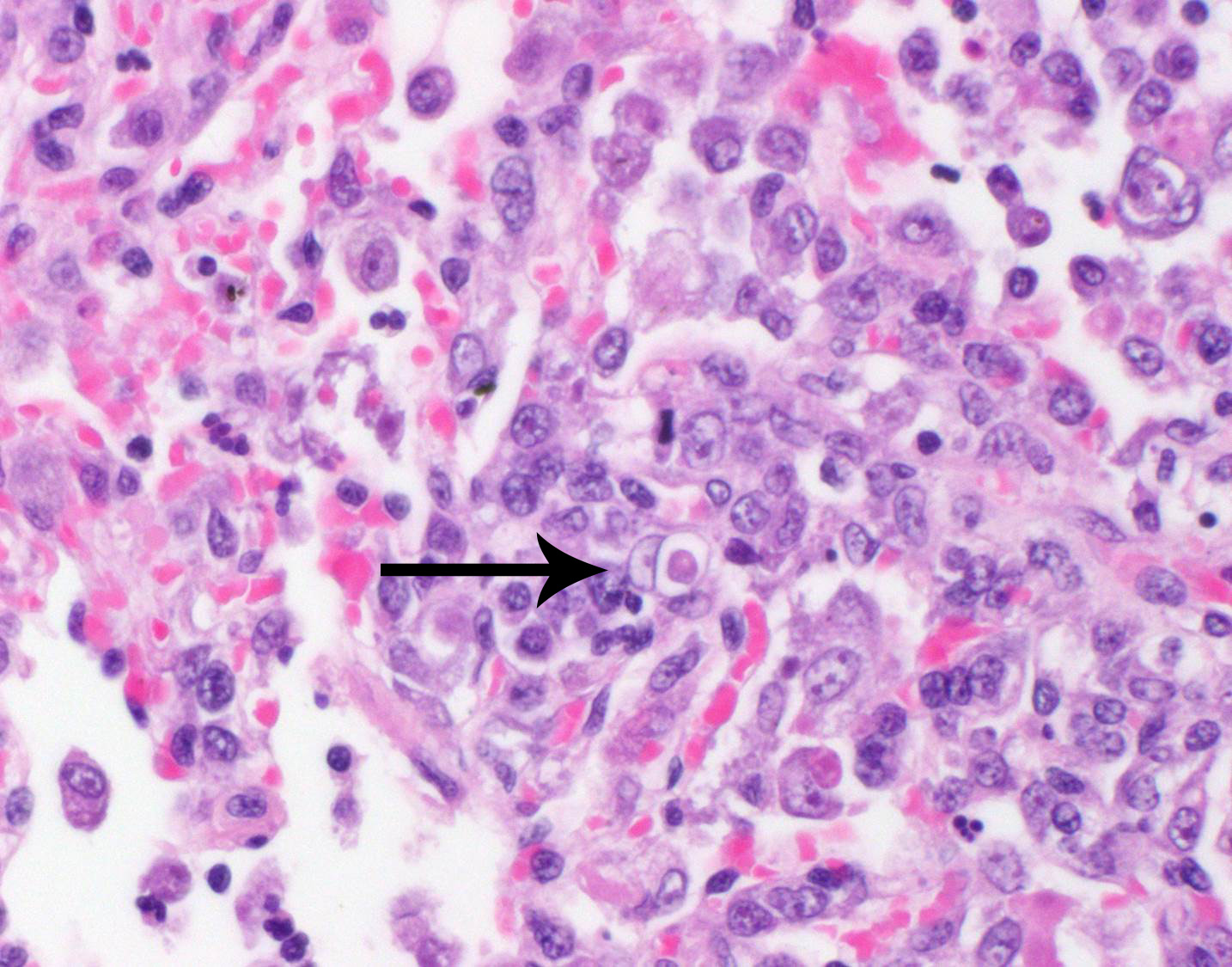
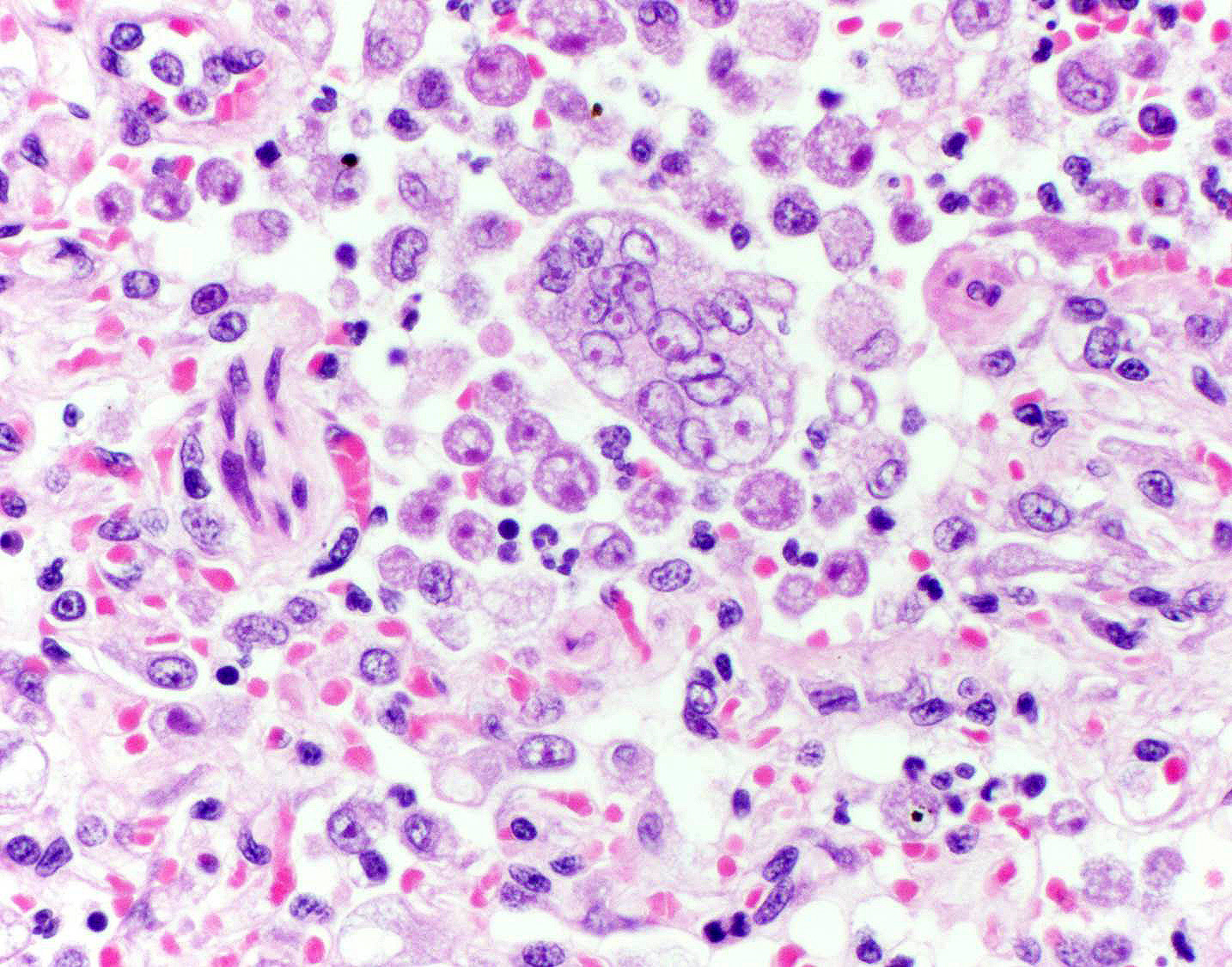
bronchioles. Within these foci are florid infiltrates of viable and degenerate
neutrophils, large foamy macrophages, and multinucleated macrophages, as well
as myriad amoebic trophozoites and rare cysts. Trophozoites are 25-30-um in
diameter, with flocculent pale eosinophilic cytoplasm and a 6-8-um karyosome
with a prominent central basophilic endosome. Cysts measure 15-20-um and have
a thick, bilayered wall (exocyst and endocyst). Within the nucleus of
macrophages (including multinucleated macrophages phagocytizing trophozoites)
adjacent to and within the most severely affected regions of the lung, there
are one to multiple prominent brightly eosinophilic intranuclear inclusions
that peripheralize the chromatin. In areas of the lung that are less severely
affected, there is filling of alveolar spaces with edema, foamy macrophages,
and low numbers of neutrophils, as well as scattered type II pneumocyte
hyperplasia, hypertrophy of vascular endothelial cells, and expansion of
residual septa by fibrin, similar inflammatory cells, and hyper-trophied
fibroblasts. There is mild multifocal mesothelial hypertrophy.
Immunohistochemistry
for canine distemper virus was negative. In-situ hybridization with a probe
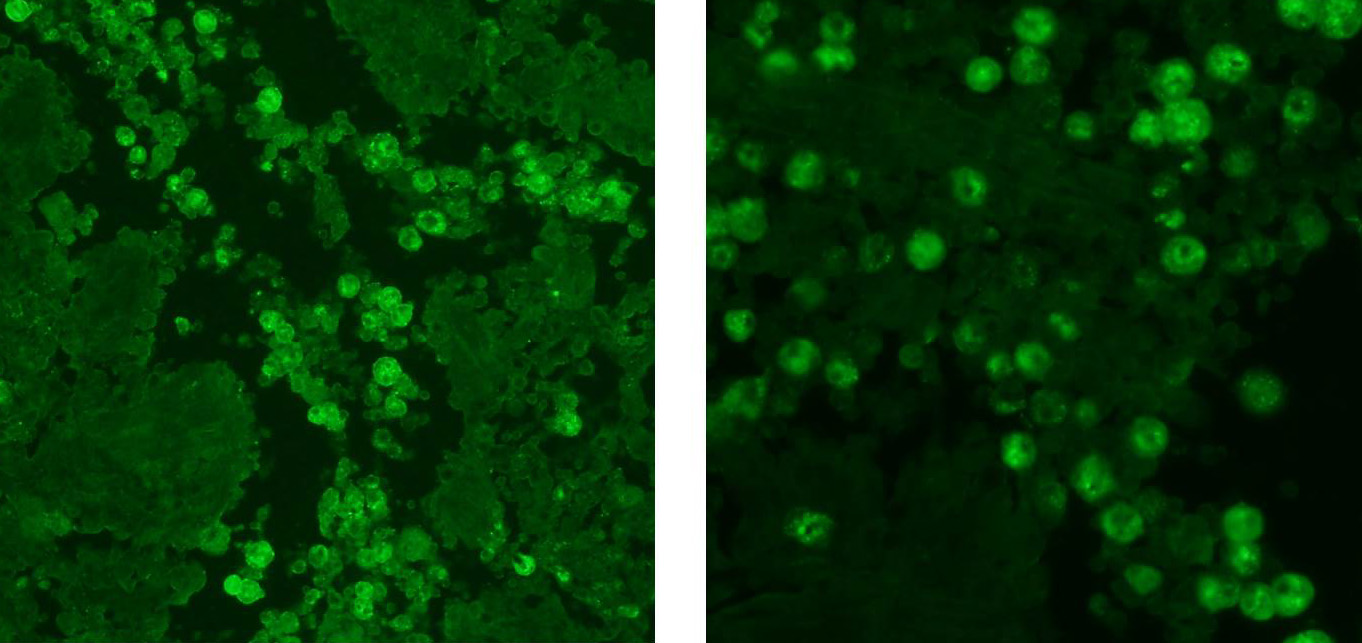
directed against canine herpesvirus was also negative. Indirect
immunofluorescence at the CDC confirmed the amoebae to be
Acanthamoeba
spp.
Morphologic Diagnosis:
Lung: Pneumonia and bronchiolitis, necrosuppurative
and hemorrhagic, multi-focal, chronic-active, severe, with free and
intrahistiocytic
Acanthamoeba spp. trophozoites and cysts.
Lab Results:
N/A
Condition:
Bronchopneumonia/Trueperella pyogenes
Contributor Comment:
Entamoeba histolytica, Sappinia diploidea, Acanthamoeba,
Balamuthia and
Naegleria species are free-living amoebas that act as secondary
decomposers and regulate bacterial population in the soil. However, they are
also opportunistic pathogens and can cause severe disseminated disease or necrotizing
granulomatous encephalitis in immunosuppressed animals and humans. In the
present case, the patient had received a short course of cyclosporine to treat
the pleural effusion, which was potentially responsible for the immune
suppression and increased susceptibility to opportunistic pathogens. Due to the
presence of both cysts and trophozoites in the lung tissues, infection with
Acanthamoeba
spp. or Balamuthia
mandrillaris was suspected.
18 Naegleria fowleri is only present as
trophozoites and does not form cysts in infected tissues.
18 However,
a definitive diagnosis of
Acanthamoeba infection was based on the
positive results of indirect immunofluorescence.
Acanthamoeba is ubiquitously
present in the environment and has been isolated from diverse sources including
sea water, beaches, pond water, soil, fresh water lakes, and even from the air.
In human populations, anti-
Acanthamoeba antibodies are present in up to
100% of people in healthy populations in New Zealand and in more than 85% of
individuals of London who came from different countries.
3,5,18 In
dogs, pulmonary infections usually result from inhalation or aspiration of the
organisms from the water.
Acanthamoeba binds to host cells by a 130 kDa mannose-binding protein (MBP)
. Other
adhesions involved in this interaction include laminin-binding protein
with a predicted molecular mass of a 28.2 kDa, a 55 kDa laminin-binding protein
and a > 207 kDa adhesion.
8,9,17 Acanthamoeba binding to
the host cells results in its phagocytosis and release of toxins. Two
superoxide dismutases, an iron superoxide dismutase and a copper-zinc
superoxide dismutase provide antioxidant defense. Toxins produced by
Acanthamoeba
cause activation of the phosphatidylinositol 3-kinase (PI3K) pathway, which
further activates pro-apoptotic molecules, Bak and Bax, resulting in loss of
mitochondrial membrane potential and apoptosis of host cells.
1,13,19
In host cells, toll-like receptor-4
(TLR4) is responsible for recognition of Acan-thamoeba , which leads to activation of the Myd88 pathway
and induces secretion of interleukin-8, tumor necrosis factor-alpha, and
interferon-beta.
13,16 Acan-thamoeba causes degradation of occludin and zonula occludens-1 tight junction proteins in
human brain microvascular endothelial cells (HBMEC) in a Rho kinase-dependent
manner, and thus leading to increased vascular permeability.
12
Acanthamoeba causes cutaneous lesions, sinus infections,
keratitis, and rare but fatal encephalitis, known as granulomatous amoebic
encephalitis in humans. Similarly,
Acanthamoeba causes encephalitis and
disseminated disease in immunosuppressed animals. In addition, most isolates
harbor endosymbionts including numerous viruses (vesicular stomatitis virus,
adenovirus, and poliovirus), bacterias (Burk-holderia spp.,Campylobacter jejuni,
Coxiella
burnetii,
Francisella tularensis,
Helicobacter pylori and Listeria monocytogenes), and yeast organisms (
Cryptococcus neoformans,
Blastomyces dermatitidis, Sporothrix schenckii, Histo-plasma capsulatum).
However, the role of endosymbionts is not entirely clear. It is suspected that
Acanthamoeba
can transmit them to susceptible hosts or endosymbionts can increase the
pathogenicity of
Acanthamoeba.11,18
The intrahistiocytic intranuclear inclusions
present in this case are strongly suggestive of a co-infection with canine
distemper virus. Unfortunately, the formalin-fixed tissue was held by the
submitting clinician for several weeks prior to submission, so it is likely
that this resulted in a loss of immunoreactivity due to antigen cross-linking.
Pneumonia, broncho-interstitial
and necrotizing, multifocal to coalescing, marked, with free and
intrahistiocytic trophozoites and rare cysts, beagle,
Canis familiaris.
JPC Diagnosis:
Pneumonia, broncho-interstitial
and necrotizing, multifocal to coalescing, marked, with free and
intrahistiocytic trophozoites and rare cysts, beagle,
Canis familiaris.
Conference Comment:
We thank the contributor for providing an outstanding and
challenging case that stimulated a great deal of discussion among the
conference participants regarding whether
Acan-thamoeba is the primary
cause of the significant pulmonary pathology in this case, or if it is
secondary to concurrent infection with canine distemper virus (CDV). Like the
contributor, many participants note numerous brightly eosinophilic intra-histiocytic
nuclear inclusion bodies within multinucleated cells, which are interpreted as
viral syncytial cells. However, others argue that the intranuclear structures
may be representative of prominent nucleoli in response to marked chronic
inflammation and the multinucleated cells are reactive fused macrophages and
megakaryocytes rather than viral syncytial cells. Participants also describe
multinucleated cells that occasionally contain phagocytosed amoebic
trophozoites.
Ionized calcium binding adaptor molecule 1 (Iba1)
immunohistochemical stain, provided by the contributor, demonstrated strong
intracytoplasmic immunoreactivity for the multinucleated cells, confirming
their macrophage lineage. The typical respiratory lesions associated with CDV
include bronchointerstitial pneumonia with prominent cytoplasmic inclusion
bodies in the bronchial and bronchiolar epithelium, type II pneumocyte
hyperplasia with alveolar cytoplasmic inclusions, and alveolar epithelial
syncytial cells.
4 Participants favoring primary
Acan-thamoeba infection
note that bronchial and bronchiolar epithelium do not contain prominent
cytoplasmic inclusions typical of CDV. Unfortunately, as mentioned by the
contributor, suboptimal tissue preservation techniques may have affected the
immunoreactivity for canine morbillivirus immunohistochemistry (IHC).
Additionally, formalin fixation dramatically reduces the ability to extract
suitable DNA for PCR based diagnostic tests. These deleterious effects of
formalin on DNA are time and concentration dependent.
14 The majority
of participants agree with the contributor that there is likely primary
concurrent infection with canine distemper virus in this case, despite negative
IHC results.
Reports of pulmonary and
systemic disease caused by free-living amoebae in dogs are uncommon and are
usually associated with immune suppression with the majority of reported cases
associated with underlying disease (such as co-infection with CDV) or long-term
immunosuppressive doses of corticosteroids
6,7,10,15; however, there
is a report of a greyhound naturally infected with
Acanthamoeba sp.
causing primary granulo-matous pneumonia and encephalitis.
2 In this
case, the dog was receiving cyclosporine to treat chylothorax after a lung
lobectomy, which may offer an alternative explanation for immune suppression;
however, the reported short course of treatment is inconsistent with the long
course of immunosuppressive therapy reported in the literature.
6,7,1
References:
1. Alizadeh H, Pidherney MS, McCulley JP, Niederkorn JY. Apoptosis
as a mechanism of cytolysis of tumor cells by a pathogenic
free-living amoeba.
Infect Immun. 1994; 62(4)
:1298-1303.
2. Bauer R.W., Harrison L.R., Watson C.W., Styer E.L. &
Chapman Jr W.L. 1993. Isolation of Acan-thamoeba sp. from a greyhound with
pneumonia and granulomatous amebic encephalitis. J. Vet. Diagn. Invest.
5:386-391.
3. Brindley
N, Matin A, Khan NA.
Acanthamoeba castellanii: High antibody prevalence
in racially and ethnically diverse populations.
Exp Parasitol. 2009;
121(3)
:254-256.
4. Caswell JL, Williams KJ. Respiratory system. I n:
Jubb Kennedy and Palmer's Pathology of Domestic Animals.
Vol 1. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:574-576.
5. Cursons RT, Brown TJ, Keys EA, Moriarty KM, Till D. Immunity
to pathogenic free-living amoebae: Role of humoral antibody.
Infect Immun.
1980; 29(2)
:401-40
6. Dubey JP, Benson JE, et al. Dissemninated
Acanthamoeba
sp. infection in a dog.
Vet Parasitol. 2005; 125:183-187.
7. Foreman O, Sykes J, et al. Dissemniated infection with
Balamuthia
mandrillaris in a dog.
Vet Pathol. 2004; 41:506-510.
8. Garate M, Cao Z, Bateman E, Panjwani N. Cloning and
characterization of a novel mannose-binding protein of Acanthamoeba.
J Biol
Chem. 2004; 279(28)
:29849-29856.
9. Hong YC, Lee WM, Kong HH, Jeong HJ, Chung DI. Molecular cloning
and characterization of a cDNA encoding a laminin-binding protein (AhLBP) from
Acanthamoeba healyi.
Exp Parasitol. 2004; 106(3-4)
:95-102.
10. Kent
M, Platt S, et al. Multisystemic infection with Acanthamoeba sp in a dog.
J
Am Vet Med Assoc. 2011; 238:1476-1481.
11. Khan NA. Acanthamoeba: Biology and increasing importance in
human health.
FEMS Microbiol Rev. 2006; 30(4)
:564-595.
12. Khan NA, Siddiqui R. Acanthamoeba affects the integrity of human
brain microvascular endothelial cells and degrades the tight junction proteins.
Int J Parasitol. 2009; 39(14)
:1611-1616.
13. Mattana A, Cappai V, Alberti L, Serra C, Fiori PL, Cappuccinelli
P. ADP and other metabolites released from
Acanthamoeba castellanii lead
to human monocytic cell death through apoptosis and stimulate the secretion of
proinflammatory cytokines.
Infect Immun. 2002; 70(8)
:4424-4432.
14. Ramos
F, Zurabian R, et al. The effect of formalin fixation on polymerase chain
reaction characterization of
Entamoeba histolytica.
Trans R Soc Trop
Med Hyg. 1999; 93:335-336.
15. Reed
LT, Miller MA, Visvesvara GS, Gardiner CH, et al. Diagnostic exercise: Cerebral
mass in a puppy with respiratory distress and progressive neurologic signs.
Vet
Pathol. 2010; 47(6):1116-1119.
16. Ren MY, Wu XY. Toll-like receptor 4 signalling pathway
activation in a rat model of Acanthamoeba Keratitis.
Parasite Immunol.
2011; 33(1)
:25-33.
17. Rocha-Azevedo B, Jamerson M, Cabral GA, Marciano-Cabral F.
Acanthamoeba
culbertsoni: analysis of amoebic adhesion and invasion on extracellular
matrix components collagen I and laminin-1.
Exp Parasitol. 2010; 126(1)
:79-84.
18. Siddiqui
R, Khan NA. Biology and pathogenesis of Acanthamoeba.
Parasit Vectors.
2012:5
-6.
19. Sissons J, Kim KS, Stins M, Jayasekera S, Alsam S, Khan NA.
Acanthamoeba
castellanii induces host cell death via a phosphatidylinositol
3-kinase-dependent mechanism.
Infect Immun. 2005; 73(5)
:2704-2708.