CASE IV: AR17-0027-7 DVD (JPC 4117660).
Signalment: 4-month-old male prairie vole (Microtus ochrogaster)
History: On the day of necropsy, this animal was found hunched, and had dried blood around the anus and on the feces. Euthanasia via C02 and cervical dislocation was performed due to poor prognosis.
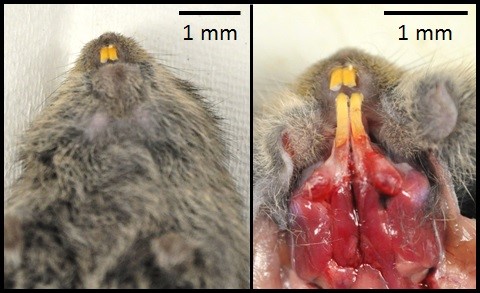
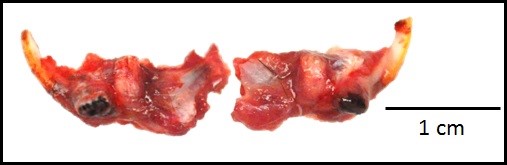
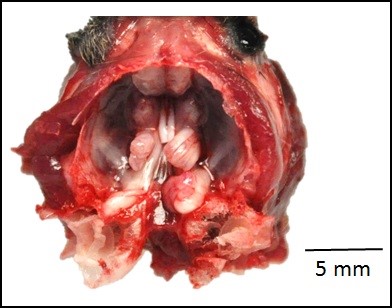
Gross Pathology: The roots of both first mandibular molars extended ventrally (2x2x1 mm) beyond the body of the mandible, and had blackened tips. The roots of both of the second mandibular molars extended from the body of the mandible, curling laterally and dorsally (3x2x1 mm). The tooth roots of maxillary molars 2 and 3 extended through the skull and into the ventral cranial vault, in the area of the pituitary gland, with the cranial pair extending in 1x1x1 mm and the caudal pair, 3x2x2 mm. An intussusception was present involving the ansa spiralis coli and proximal colon.
Laboratory results: NA.
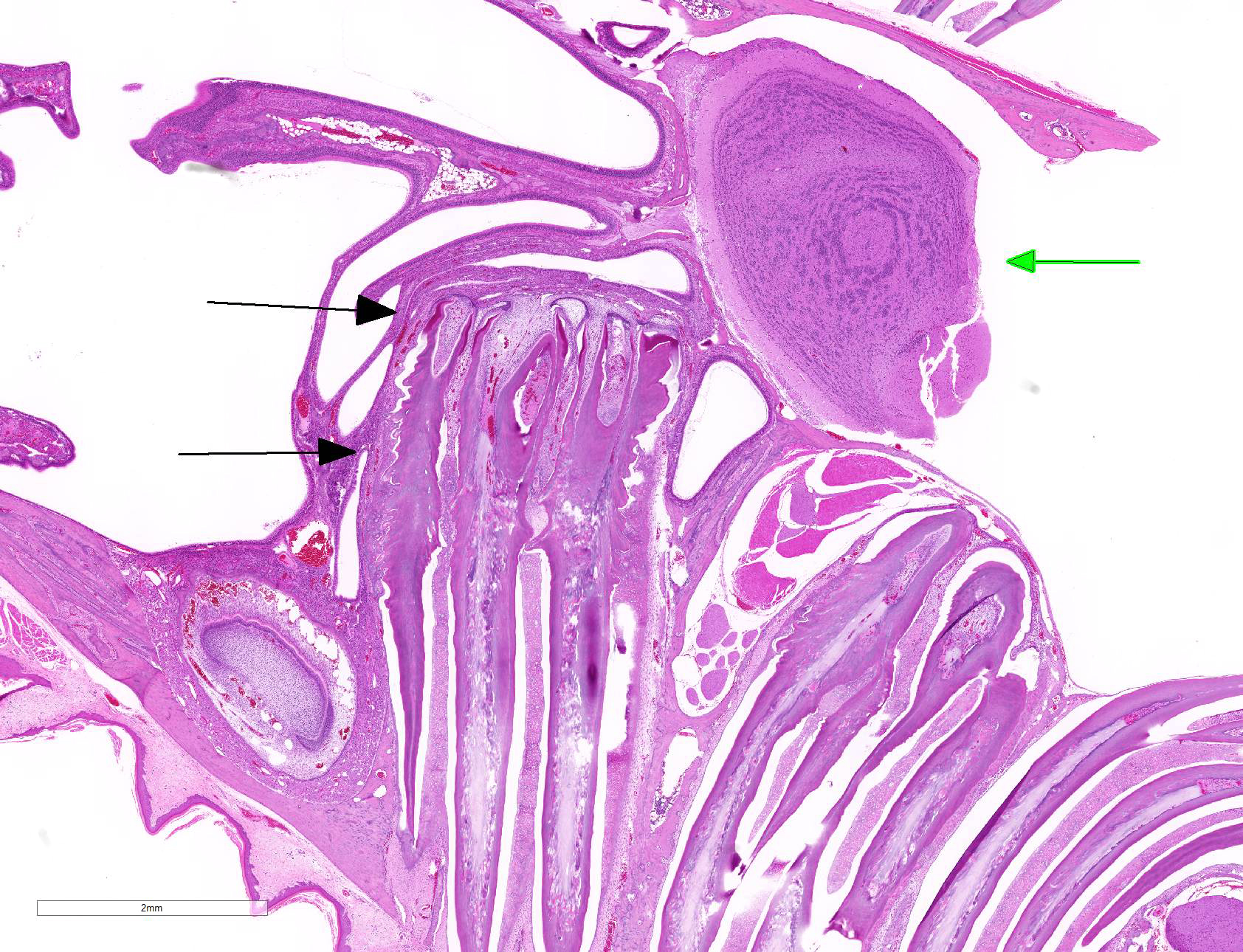
Microscopic Description:
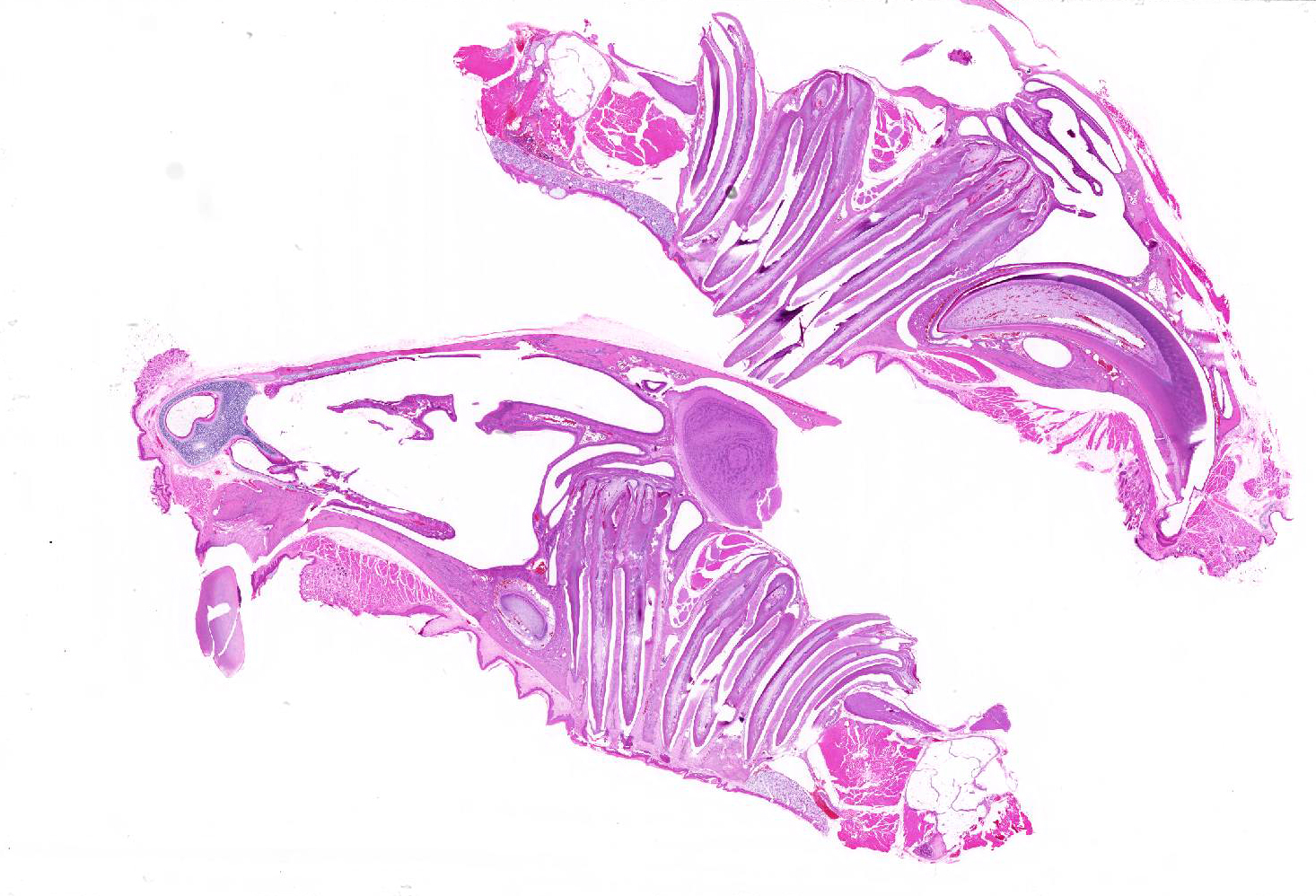
Sagittal section of the skull: The molar roots extend cranially, the first molar (M1) compresses the nasal sinuses, and the second and third molars (M2 and M3) extend into the cranial vault. The pulp cavities and dentin have irregularly scalloped edges, and the stellate reticulum is retained. The cortical bone overlying the molars is thin, irregularly scalloped, and has many smooth (resting) and haphazard (reversal) basophilic lines throughout. The bone is lined by closely spaced osteoblasts and fewer osteoclasts in Howship?s lacunae, and some osteocytes are pale without cellular detail, or are not present in lacunae (necrosis).
Contributor Morphologic Diagnosis:
Dental dysplasia with invasion of the cranium, maxillary molars
Degeneration and regeneration, multifocal, chronic, moderate, maxilla
Contributor Comment: This animal was one of sixteen cases of laboratory prairie voles (Microtus ochrogaster) at our facility which presented with varying degrees of molar apical elongation in the mandible and/or maxilla. The animals ranged from 4 to 13 months old, and both genders were represented. All cases had bilateral mandibular molar roots that extended apically beyond the body of the mandible, and fourteen voles had maxillary molars which extended apically through the skull into the cranial vault and compressed the nasal sinuses. The histologic findings for all of these animals revealed dental dysplasia with abnormal dentin, irregular pulp cavities, and marked remodeling of the surrounding bone.
There was neither apparent correlation between age and the size of the mandibular root protuberances, nor between the size and any clinical sign. Six prairie voles had oral lesions (abscessation, food impaction, ulceration, osteomyelitis) which were attributed to the dental deformity, three had concurrent coronal incisor overgrowth, and two died of sepsis secondary to oral infection. The brain was unaffected in all but three cases where cerebral compression was noted.
Prairie voles have aradicular hypsodont dentition10, which displays continual growth of teeth throughout life, offsetting the wear incurred from a fibrous diet. Other terms for this pattern of growth are hypselodont or elodont dentition.8 Unlike other rodents including mice (Mus musculus) which have only continual growth of the incisors, all vole teeth, including the one incisor and three molars in each quadrant, continually grow. Other animals with complete aradicular hypsodont dentition include rabbits, guinea pigs, and chinchillas.12 It has been proposed that the persistence of the stellate reticulum plays a role in the perpetual tooth growth in the incisors of mice and vole teeth.8 The stellate reticulum is a stem cell niche within the cervical loop of the proximal tooth which sits between the inner and outer enamel epithelia.9 Epithelial stem cells are thought to be located within the stellate reticulum and enamel epithelium (also known as Hertwig?s epithelial root sheath) at the edge of the cervical loop. Stellate reticulum stem cells migrate to the inner enamel epithelium and support proliferating cells which then relocate distally to differentiate into ameloblasts.14 In mice, the stellate reticulum of the molars regresses during maturation, explaining the cessation of growth. The regulation of growth in mouse incisors and the molars of sibling voles (Microtus rossaiemeridionalis) have been shown to be under the influence of FGF10, Sox2+ and Notch signaling, in addition to potential influence of BMP4.2,14 The histology presented from this case showed retention of the stellate reticulum in the maxillary molars of an adult vole with dental dysplasia. Normal teeth from unaffected adult animals in this cohort also had retained stellate reticulum.
Similar findings in a colony of prairie voles at another facility have been studied, and it was speculated that a spontaneous mutation in dental stem cell regulatory genes was responsible for the condition. The inheritance was considered to be complex, as experimental inbreeding of affected animals produced no gross molar changes in the F2 offspring by 8-12 months of age.8 In both this study and observations from our cases some animals were lethargic, moribund, or found dead, which was thought to have been attributable to the invasion of the skull and compression of the brain, although a definitive link could not be established.
A number of Microtus species (including M. californicus californicus, M californicus vallicola, M. montanus, M. pennsylvanicus, and M. socialis) have been reported with this abnormality4. Molar apical elongation in a colony of captive Amargosa voles (Microtus californicus scirpensis) was attributed to clinical signs including abnormal mentation, ocular discharge and abnormal mastication. Histology of these lesions showed variable hyperplasia, dysplasia, and atrophy of the inner and outer enamel epithelial layers.4 A Japanese field vole (Microtus montebelli) from one study presented with maxillary molar macrodontia that protruded apically and invaded the brain as deep as the thalamic nuclei.13 Coronal molar overgrowth without incisor abnormalities has been reported in a colony of laboratory pine voles (Microtus pinetorum). Changes in the husbandry of the pine vole colony, which included increasing dietary roughage and adding hard wood as enrichment, appeared to prevent further occurrence of this lesion.3
Although the stellate reticulum was also retained in the incisors of the adult voles in our cases, none of the incisors displayed apical elongation. This suggests that the cause of the dysplasia is more complex than simply a mutation in the stellate reticulum, or perhaps the incisor and molar stem cell niches are dissimilar. Another supposition is the possibility that a causative mutation may only affect the molar-specific mechanic and transduction pathway, or there may be differences in the periodontal ligament anchorage between incisors and molars.8 Some studies have suggested that the cause of molar apical elongation is multifactorial, including genetic predisposition, deficient occlusal attrition and environmental factors such as inadequate roughage in the diet.4
A colony of Steppe lemmings (Lagurus lagurus), which are aradicular hypsodonts in the same subfamily as voles, had apical odontogenic dysplasia of the mandibular and maxillary molars with cranial vault invasion. In contrast, on histology, the apical overgrowths were composed of highly disorganized mature odontogenic structures, and more closely resembled hamartomas, which in aradicular hypsodonts have been termed elodontomas5.Changes comparable to molar apical elongation and elodontoma formation occur in many aradicular hypsodont animals, including rabbits, squirrels, and degus.1,6,7 These findings indicate that some degree of apical molar overgrowth is intrinsic in species with this dentition.4
Contributing Institution:
Wake Forest School of Medicine
Department of Pathology, Section on Comparative Medicine
Medical Center Boulevard, Winston-Salem, NC 27157
www.wakehealth.edu
JPC Diagnosis: Head, sagittal section, cheek teeth: Odontogenic dysplasia with alveolar bone remodeling, and penetration of the maxillary sinus and calvarium.
JPC Comment:
The contributor has given us an excellent review of dental growth in rodents as
well as the available literature on dysplastic teeth lesions in the rodent.
Hyperplasia and dysplasia with apical elongation appears to be a considerable
problem in voles of the genus Microtus. The changes noted in this
particular case are similar to those which were well-described in a captive
colony of Amargosa voles (Microtus californicus scirpensis) by Imai et
al.4
In these particular voles the lesion of molar apical elongation (MAE) was
characterized by a combination of hyperplasia and to a lesser extent dysplasia
which was seen in 63% of the colony. The single predisposing factor in this
particular report was age. The authors believe the entity to be multifactorial
as a result of a) the nature of continuous growth of hypsodont teeth,
inadequate occlusal attrition, and c) a possible genetic inheritance.
That particular paper also describes, with excellent photomicrographs, many of
the changes seen in this condition. While it has often been referred to as
dentinal dysplasia in rodents, the disorganization of elements seen in true
odontogenic dysplasia is not evident in this particular sample other than mild
compression of the intercuspal loops and scalloping of the dentin, so we prefer
the overall diagnosis of hyperplasia as described by Imai et al. than dysplasia
in this particular 4-month-old individual. It might be true that this lesion
might become more dysplastic over time as the animal matured.
The term odontogenic dysplasia has been applied to a number of proliferative masses in rodents and lagomorphs over the years, and used interchangeably with odontoma, pseudo-odontoma, and elontoma (all of which incorrectly imply a neoplastic origin.) The etiology of these masses is likely to be disruption of the embryonic enamel organ by a variety of means, including irritation, microtrauma, or infection. It is characterized by a disorganized mass of odontogenic epithelium with significant mineralized dental matrix (dentin, enamel, and in the case of hypsodont teeth, cementum.)11
References:
1. Boy SC, Steenkamp G. Odontoma-like tumours of
squirrel elodont incisors?elodontomas. J Comp Pathol. 2006;135(1):56?61.
2. Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization
of putative stem cells in dental epithelium and their association with Notch
and FGF signaling. J Cell Biol. 1999; 147(1): 105?120.
3. Harvey SB, Alworth LC, Blas-Machado U. Molar malocclusions in pine voles (Microtus
pinetorum). J Am Assoc Lab Anim Sci. 2009; 48(4): 412?415.
4. Imai DM, Pesapane R, Conroy CJ, Alarcón CN, Allan N, Okino RA, Fung J,
Murphy BG, Verstraete FJM, Foley JE. Apical Elongation of Molar Teeth in
Captive Microtus Voles. Vet Pathol. 2018 Jul;55(4):572-583. doi:
10.1177/0300985818758469. Epub 2018 Apr 17.
5. Imbschweiler I, Schauerte N, Henjes C, Fehr M, Baumgärtner W. Odontogenic
dysplasia in the molar teeth of Steppe lemmings (Lagurus lagurus). Vet
J. 2011 Jun;188(3):365-8.
6. Jekl V, Hauptman K, Skoric M, et al. Elodontoma in a degu (Octodon degus).
JExot Pet Med. 2008;17(3):216?220.
7. Jekl V, Redrobe S. Rabbit dental disease and calcium metabolism--the science
behind divided opinions. J Small Anim Pract. 2013 Sep;54(9):481-90. doi:
10.1111/jsap.12124.
8. Jheon A, Prochazkova M, Sherman M, Manoli DS, Shah NM, Carbone L, Klein O.
Spontaneous emergence of overgrown molar teeth in a colony of prairie voles (Microtus
ochrogaster). Int J Oral Sci. 2015 Mar; 7(1): 23?26.
9. Juuri E, Saito K, Ahtiainen L, Seidel K, Tummers M, Hochedlinger K, Klein
OD, Thesleff I, Michon F. Sox2+ stem cells contribute to all epithelial
lineages of the tooth via Sfrp51 progenitors. Dev Cell. 2012; 23(2): 317?328.
10. Mushegyan V, Eronen JT, Lawing AM, Sharir A, Janis C, Jernvall J, Klein OD.
Continuously growing rodent molars result from a predictable quantitative
evolutionary change over 50 million years. Cell Rep. 2015 May 5;11(5):673-80.
11. Murphy BG, Bell CM, Soukup JW. Odontogenic dysplasia. In: Veterinary
Oral and Maxillofacial Pathology. Hoboken, NJ: Wiley and Sons, 2020: pp.
39-41.
12. Renvoisé E, Michon F. An Evo-Devo perspective on ever-growing teeth in
mammals and dental stem cell maintenance. Front Physiol. 2014 Aug 28;5:324
13. Sugita S, Uchiumi O, Fujiwara K, Niida S, Fukuta K. Brain deformation
caused by hyperplasia molar teeth (macrodonts) in the Japanese field vole (Microtus
montebelli). Exp Anim. 1995 Oct;43(5):769-72.
14. Tummers M, Thesleff I. Root or crown: a developmental choice orchestrated
by the differential regulation of the epithelial stem cell niche in the tooth
of two rodent species. Development. 2003 Mar;130(6):1049-57.