WSC 22-23:
Conference 1
CASE III:
Signalment:
10-month-old calf, female, Limousine, Bovine (Bos taurus taurus).
History:
A flock of 70 calves enters the feedlot at the beginning of August 2020. Soon after, these animals began to suffer severe problems (such as conjunctivitis, dyspnea and sudden deaths) that lasts until November. Before death, the animal suffered severe dyspnea for a week.
Gross Pathology:
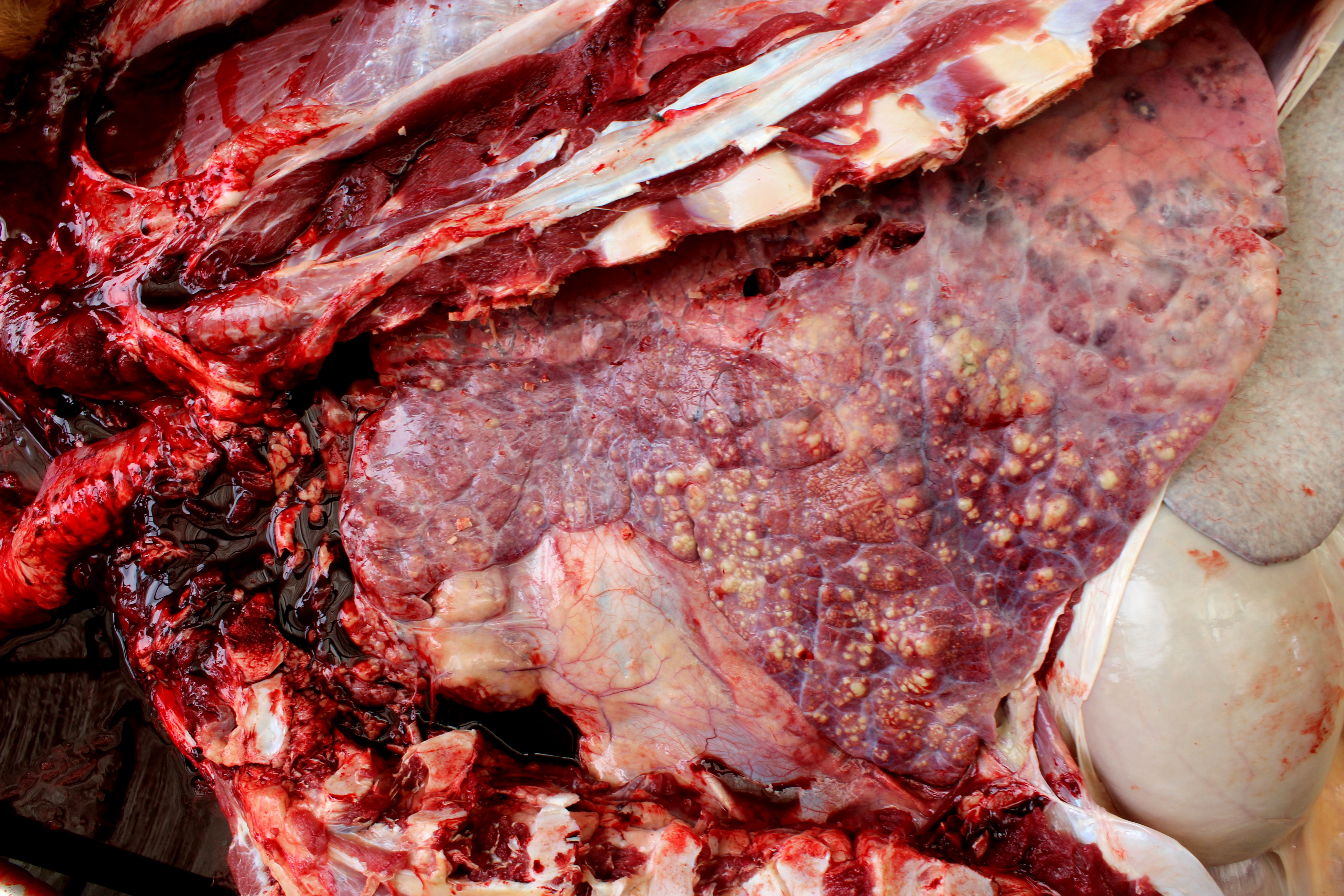
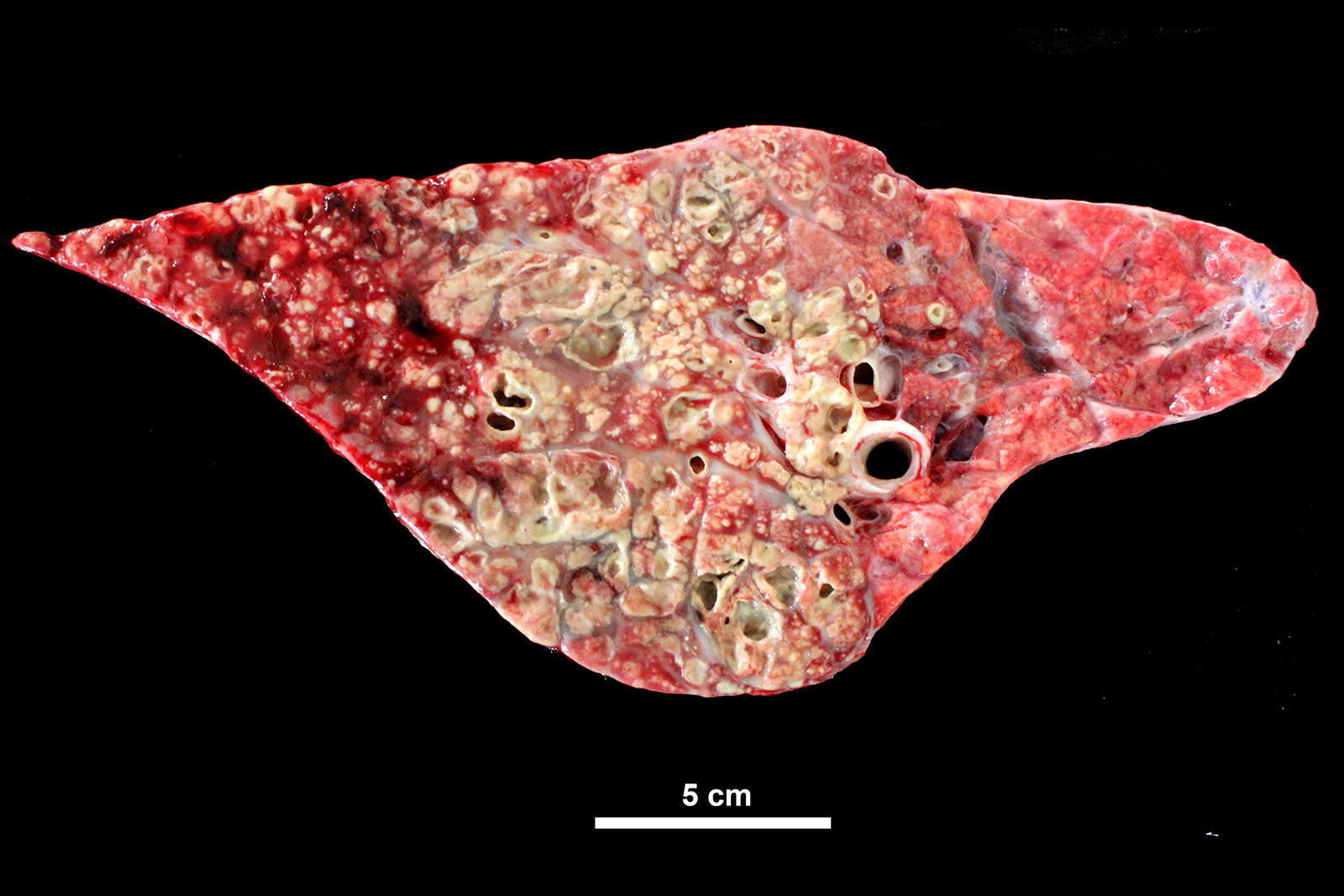
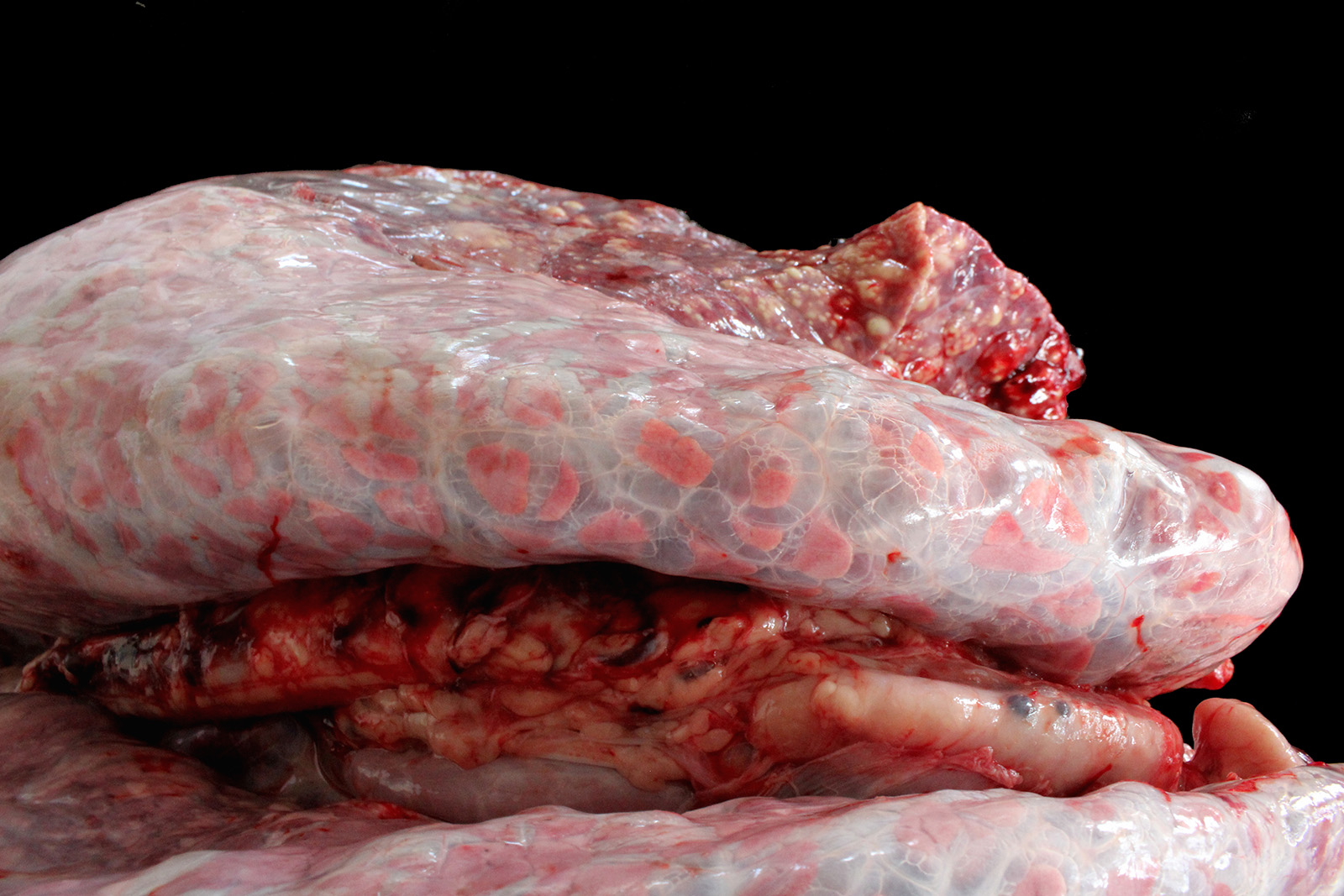
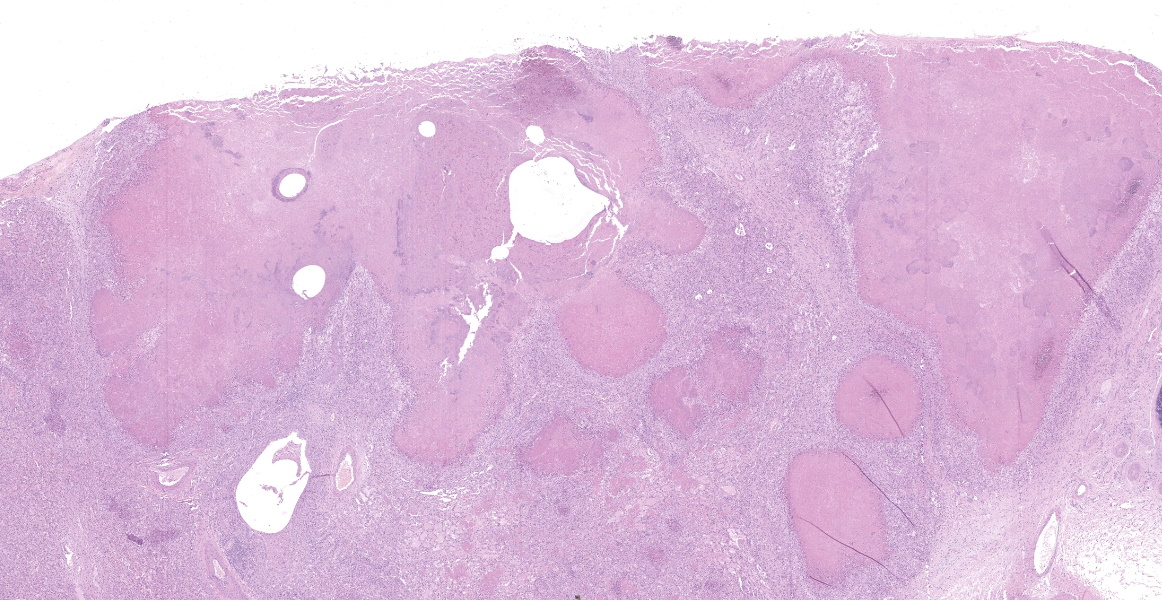
Lung: cranioventral to caudal chronic, severe, pulmonary consolidation with multifocal to coalescing, well-demarcated and variably sized pale yellow and slightly raised nodules (caseonecrotic bronchopneumonia). On cut, these lesions with caseous material extend through parenchyma and appear within distended airways, such in small bronchioles and alveoli, accompanied by areas of hemorrhage. Along the dorsal aspect of the lung, there was a severe emphysematous distension and thickening of lobular septae (interstitial emphysema). On cut surfaces, these septae appeared thickened and
edematous with small gas bubbles admixed.
Laboratory Results:
PCR positive for Mycoplasma bovis.
See Table 3-1
|
Table 3-1: Laboratory results |
|||
|
SAMPLE |
TEST |
AGENT |
RESULT |
|
Fresh lung |
Culture and mass spectrometry technology (MALDI-TOF) |
Mannheimia haemolytica |
Positive ++ |
|
Fresh lung |
Culture and mass spectrometry technology (MALDI-TOF) |
Trueperella pyogenes |
Positive +++ |
|
Fresh lung |
Culture and mass spectrometry technology (MALDI-TOF) |
Escherichia coli |
Positive +++ |
|
Pool of 2 samples of fresh lung |
Real time PCR |
Pestivirus |
Positive |
|
Pool of 2 samples of fresh lung |
Real time PCR |
IBR |
Positive |
|
Pool of 2 samples of fresh lung |
Real time PCR |
Parainfluenza 3 |
Positive |
|
Pool of 2 samples of fresh lung |
Real time PCR |
BRSV |
Negative |
|
Pool of 2 samples of fresh lung |
Real time PCR |
Bovine coronavirus |
Negative |
|
Pool of 2 samples of fresh lung |
Real time PCR |
Mycoplasma bovis |
Positive |
|
Pool of 2 samples of fresh lung |
Real time PCR |
Pasteurella multocida |
Positive |
|
Pool of 2 samples of fresh lung |
Real time PCR |
Mannheimia haemolytica |
Positive |
|
Pool of 2 samples of fresh lung |
Real time PCR |
Histophilus somni |
Negative |
|
Pool of 2 samples of fresh lung |
Real time PCR |
Birbestenia trehalosi |
Negative |
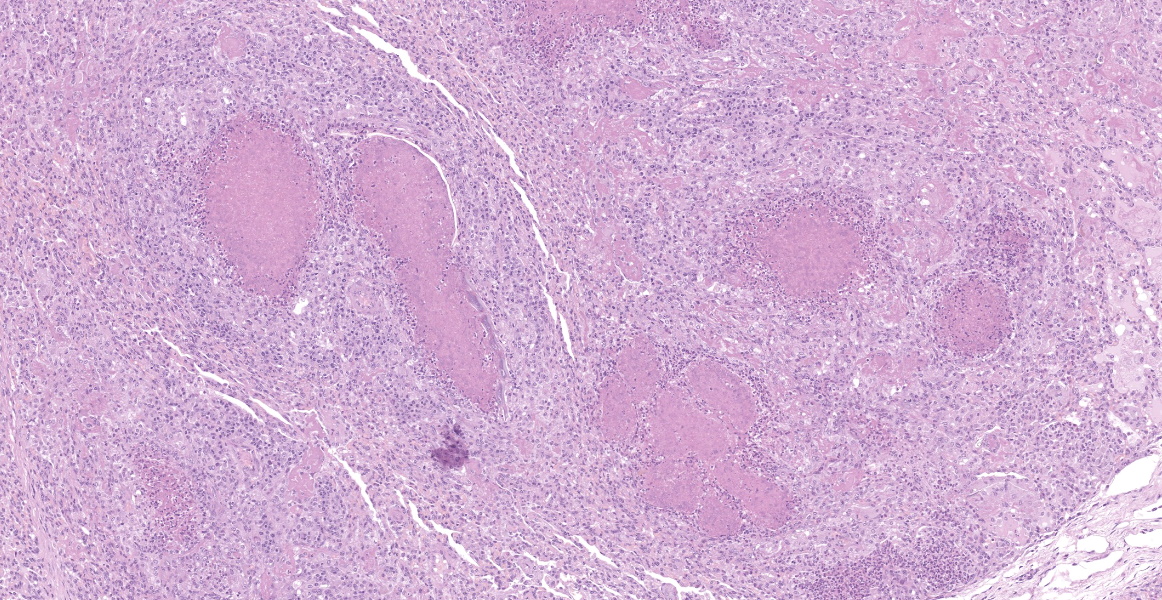
Microscopic Description:
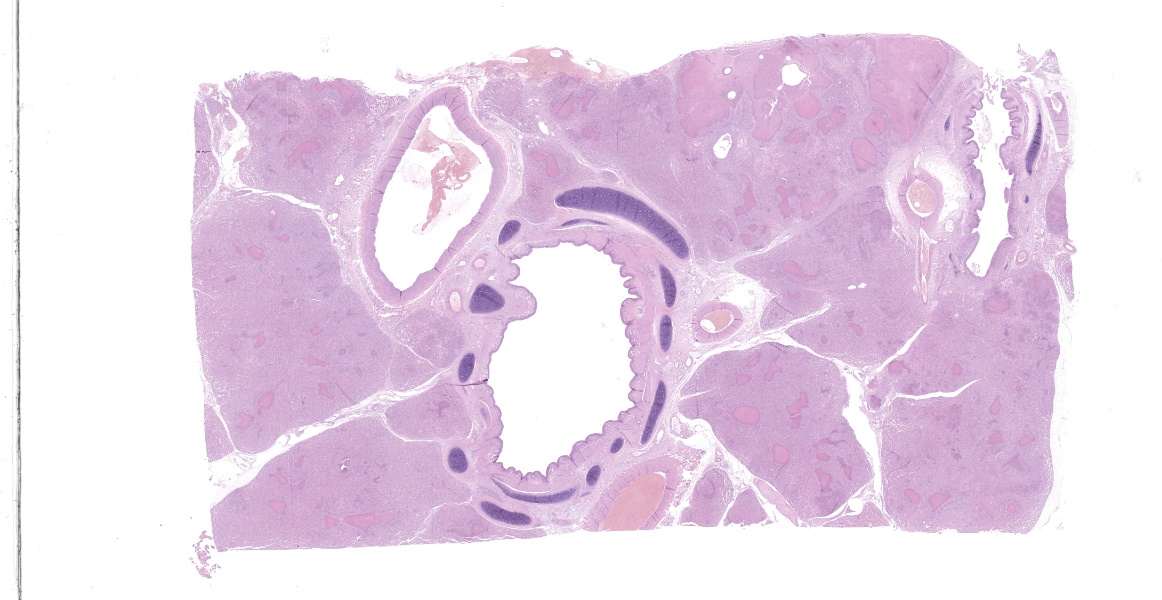
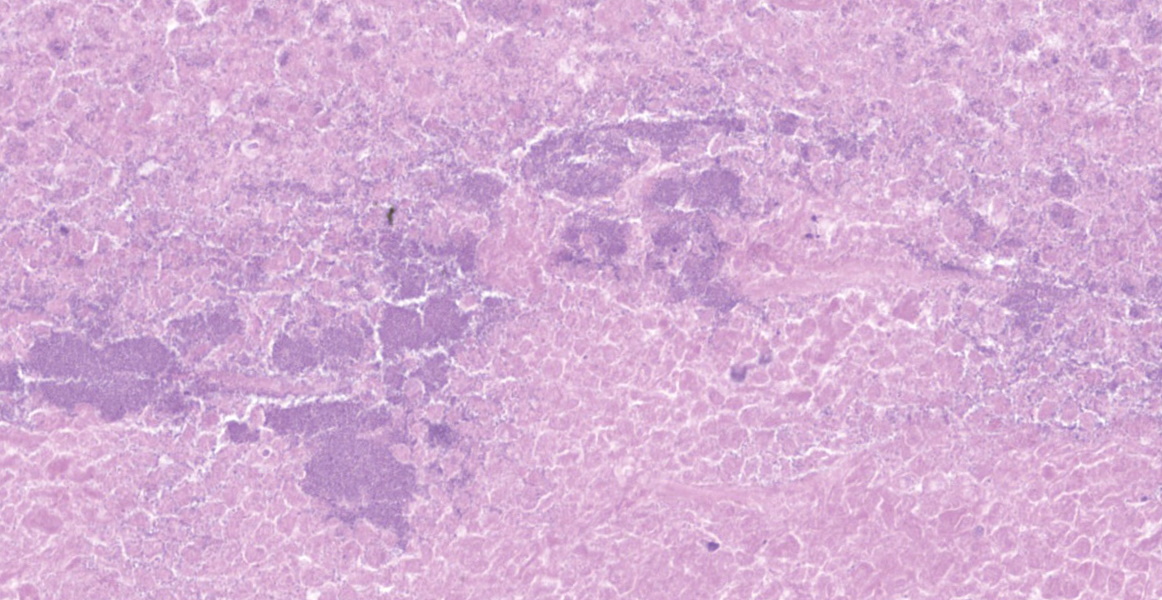
Lung: a lesion of inflammatory origin is diffusely obliterating respiratory spaces throughout the whole section of the lung, with extensive multifocally necrotizing coalescing foci centered mainly in bronchioles and alveoli. These foci are composed by an eosinophilic material with a mild admixed cellular debris delimited by a variable number of devitalized leukocytes that maintain cellular figures (ghost-like remnants of leukocytes) and a small number of active macrophages with scattered lymphocytes and some fibroblasts (caseonecrotic centers). Other bronchioles and alveoli are obliterated by high number of viable neutrophils (initial lesions). Sometimes they have multifocal mineralized areas and have multiple distributed clusters of basophilic cocci to coccobacilli bacterial colonies mainly at the periphery, but also
admixed with the caseonecrotic debris. Occasionally, there are also inside, foreign bodies most compatible with inhaled vegetable structures or hair. Sometimes, these exogenous particles contain bacterial colonies or are mineralized. There are small, scattered foci with leukocytes with streaming hyperchromatic to smudgy nuclei fill the alveoli (oat cells) with fibrin, edema and necrotic karyorrhectic debris with no centers of caseous necrosis. Main bronchi epithelium is hyperplasic with some degenerative changes, individual apoptotic cells and mild submucosal lymphoplasmacytic infiltrate and reactive BALT. In the rest of pulmonary parenchyma alveolar septa are thickened by a marked hyperplasia of pneumocytes type II, macrophages, neutrophils, red blood cells and some fibrin thrombi. Alveolar spaces have areas containing eosinophilic and fibrillar agglomerated substance (polymerized fibrin) and unsettled areas of homogenous eosinophilic clear fluid (edema). Others have severely number of macrophages sometimes phagocyting fibrin and occasional multinucleated cell of 2-7 nuclei. Some arterioles and small arteries within the parenchymal tissue have extended walls with muscular layer hypertrophy and fibrin deposition. Other major arteries present intraluminal thrombi and some mononuclear inflammatory cells. Pleura and pulmonary septae around main arteries are distended by edema and some mononuclear cells that also distend some lymphatic vessels.
Contributor's Morphologic Diagnoses:
Lung: Severe chronic multifocal to coalescing caseonecrotic and necrotic bronchopneumonia with intralesional colonies of bacterial cocci.
Lung: Diffuse interstitial pneumonia with pneumocytes type II hyperplasia, fibrin and interlobular edema.
Contributor's Comment:
Mycoplasmas were discovered for the first time by the team of Pasteur, Nocard and Roux in 1898, when the first report of mycoplasmosis was released 35. This mycoplasma was isolated from a cattle and was compatible with the current mycoplasma mcyoides subsp mycoides (formerly known as M.mycoides subsp. mycoides 'small colony'). At that time mycoplasma organisms were known as pleuropneumonia like-organisms (PPLO) for being the causative agent of contagious bovine pleuropneumonia (CBPP) 33,35. Nowadays these microbes belong to the genus Mycoplasma spp. and the class Mollicutes, which differ from other bacteria by their small genomes (580-2220 Kb) and no cell wall 44. Mycoplasmas are highly contagious organisms and their virulent spread throughout Europe and the world during middle 19th century by cattle trade is well documented. The infection was controlled by stamping out policies, persisting in African countries during 20th century 14,36.
Currently Mycoplasma bovis is one of the major causative agents of bovine mycoplasmosis 7,8 and is also consider a major player in bovine respiratory disease complex (BRD) 3. It was first isolated in 1961 in the United States 13, since then, has been turned to be the one of the more pathogenic species of the genus Mycoplasma9. This has not been enough to implement restrictions on cattle movement related to M. bovis37. M. bovis has a well-known economic, productivity, welfare and health impact in dairy and beef cattle worldwide 32, particularly on the North America and more recently in continental Europe 1. This impact is due to the frequent chronicity of the infection 10,12,32, the increasingly unresponsiveness to most treatments due to antibiotic resistance and this chronicity 28,31 and the variety of clinical manifestations including most commonly bronchopneumonia2,8,11,16,18,19,42, otitis media16,27, mastitis1,6,8,15,20,32,36,43 and arthritis/tenosynovitis19,42. Other less consistent manifestations include urogenital disorders (metritis, abortion, infertility, endometritis, salpingitis, reduction of conception rate, seminal vesiculitis, epididymitis and orchitis in bulls) 6,11,23, meningitis4, keratoconjunctivitis 2,29, decubital abscesses 26 and endocarditis25.
The incubation period is difficult to define because it varies with the age and these clinical and pathological effects 9. The clinical expression of these manifestations is highly variable and there are individuals without clinical symptoms in which the organism is isolated from the upper respiratory tract (URT). Thus, the presence M. bovis not always result in disease 21,32,43. As Trojan horses, these asymptomatic individuals are the leading cause of introduction of M. bovis into disease-free herds and the maintenance of infection. These animals can shed the bacteria intermittently from a few months to several months or even years 11,32. Probably, this particular case and the flock were naive at the moment of entry to the chronic infected feedlot. In fact, stressors related to immunosuppression such as transportation, entry of a new animal, overcrowding and weaning increase the shedding of the bacteria 9,12,32,37.
The mammary gland and the mucosa of the upper respiratory tract (URT) have been shown to be the most serious sites of persistence and transmission. It is transmitted through secretions of the UPR, through milk, from udder to udder, by ingestion and inhalation of aerosols in the case of the calf and through close contact between animals. Infrequently, colostrum, genital secretions, and fomites are a source of infection 11,13,21,32,37. The possible intrauterine route has also been discussed 22.
Endowed with some proposed virulent mechanisms, M. bovis evades the host immune response resulting in chronic infections 9. However, the pathogenesis is not fully understood and this and other molecular mechanisms involved are still under study7. Great advances have been made in this regard with the improvement of genetic techniques, such as the complete sequencing of the M.bovis genome 34,45. Certain high-frequency rearrangements of its genome give it an ability to variably express the surface Vsp lipoprotein and avoid the host immune response. Other mechanisms are adhesion to host cells with adhesins, through surface Vsp, or even metabolic enzymes such as a-enolase, NADH oxidase, TrmFO protein and the in vitro discovered fructose 1,6-biphosphate aldolase and methylenetetrahydrofolate-tRNA-(uracil-5-)-methyltransferase. M. bovis has nucleases (such as MBOVPG45, MnuA and MBOV_RS02825) that can destroy NETs and also cause macrophage apoptosis. Other form of immunologic evasion would be by intracellular infection of epithelial cells, red blood cells and circulating immune cells 9,37. The formation of a protective biofilm continue to be studied 9,32,37 and Vsp not only mediates adherence, but strongly induces immune response through activation of TLR2 and IL-1b production 9. The production of H2O2 as part of its virulence is suspected 9,37.
The most common gross lesions described for M. bovis are those observed in this case characterized by cranioventral lung consolidation and multifocal to coalescing caseonecrotic nodules that varies from pinpoint to several centimeters. They are well-demarcated, coalescing, circular, white, dry, crumbly, bulging from the pleural or cut surfaces of the lung. In addition, these nodules are delimited by areas of reddened, collapsed and consolidated lung, and the majority of nodules can be better observed with a cut through the parenchyma. Normally, these lesions are bilateral and can affect the 20% to 90% of the lung in severe cases, extending from cranioventral lobes to medial and even with relative sparing of the caudal and dorsal aspects of the caudal lobes. The key feature of this diagnosis is the friable caseous nature of the lesions, but sometimes can appeared liquefied with a suppurative consistence because secondary contamination of nodules with other bacteria (such as Trueperella pyogenes) 11,12. Occasionally we can also observe foci of coagulative necrosis that differ from caseonecrotic nodules, because they are irregular in shape, red tan, non-friable and not raised 11. The secondary interstitial emphysema observed in the dorsal aspect of the lung may be associated with the one-way valve effect of pulmonary exudates and/or the high pressure of air within the lung caused by the severe dyspnea 30. Infection with M. bovis by itself could be involved as a neutrophils elastase has been related with emphysema 17,30 and M. bovis can induce greater secretion of theses proteases by neutrophils 24.
Histologic lesions include four main patterns of lesions: caseonecrotic bronchopneumonia (most common), bronchopneumonia with foci of coagulation necrosis, suppurative bronchopneumonia without necrosis and chronic bronchopneumonia with abscessation. Caseonecrotic nodules are distinctive lesions with caseous necrosis that fill small bronchioles, alveoli or interlobular septa. As we could observed on tissue section, in the earliest lesions, leukocytes are within the airways, but undergo a distinctive form of necrosis maintaining their ghost-like cellular outlines, having hypereosinophilic cytoplasm with inapparent or fragmented nuclei. The respiratory epithelium appeared eroded and this nodules are delimited by layers of necrotic cells with pyknotic nuclei, macrophages, lymphocytes and fibroblasts 11,12.
Coagulative necrosis could coexist and complicate lesions. We could observe scattered patterns of this necrosis in which bronchiolar or alveolar structure remains visible. M. bovis could be related with those patterns sometimes, but they are indistinguishable from Mannheimia haemolytica. The suspicion of Mannheimia haemolytica coinfection was confirmed by PCR and its presence could be related with those areas of the so called oat cells, that are not expected in mycoplasmosis 11; elongated hyperchromatic cells, with the appearance of being perforated and smudged. This is a result of the activity of the typical ruminant specific Mannheimia haemolytica leukotoxin (Lkt), which induces multiple transmembrane pores in the macrophages membrane at a high concentration, and induces bovine cells to undergo respiratory burst and release of inflammatory mediators and cytokines at a sublytic concentrations 5,40. In fact, bacterial coinfections with mycoplasma are common in cases of pneumonia 18,19,32,42 and even otitis 27,32. On one occasion, M. bovis was isolated from 82% of feedlot calves with fibrinosuppurative pneumonia from which Mannheimia haemolytica was isolated. 19,32. Other bacteria such as Pasteurella multocida (isolated in this case) or Histophilus somni have been described 9,13,18,37, so bacterial culture should be done in order to identificate coinfective organisms. Mycoplasma exhibits a very slow growth up to 10 days in culture, which is why PCR is the preferred method for confirming the pathogen 36. Concomitant infections with other viruses such as Bovine viral diarrea virus (BVDV), Infectious bovine rinothracheitis virus (IBRV) and Bovine parainfluenza 3 virus (bPI-(3)V) were detected. The relationships with viral coinfections and Mycoplasma is less clear 34, but these and other viruses including BRSV (Bovine respiratory syncytial virus), bovine adenoviruses (BdVs) or bovine coronavirus (BCV) have been isolated. These viruses have been frequently detected with M. bovis and their possible synergistic role in the disease has been extensively discussed
9,10,18,32,37,42. However, it exists some discrepancies about it 38.
Recently, M. bovis, which had been anecdotally described in North American bison from 2000, was finally detected in an outbreak affecting bisons of all ages. In this case, M. bovis bison strain (most probably a host adapted variant) showed different genetics to that isolated from cattle and, unlike bovines, the organism was the primary agent of a process that had a mortality of up to 45% 37,39.
Contributing Institution:
Universidad de Zaragoza. Departamento de Patologia Animal
https://patologiaanimal.unizar.es
JPC Diagnosis:
Lung: Pneumonia, fibrinosuppurative, caseating and necrotizing, diffuse, severe, with bronchiectasis and colonies of coccobacilli.
JPC Comment:
The contributor provides a thorough report on the pulmonary manifestation of Mycoplasma bovis as well as the history, virulence factors, control, and ongoing research for this economically important disease. During the conference, the moderator emphasized the importance of the muco-ciliary apparatus in protecting the lung and described a list of pathogens which can cause dysfunction, including Mycoplasma, Bordetella, viruses, and dehydration. Mucociliary apparatus dysfunction is an important part of the pathogenesis of bovine respiratory disease complex, a disease which costs $1B USD annually in the United States alone.
As the contributor mentions, M.bovis can cause a spectrum of diseases, and a brief review of the other most common manifestations is worthwhile. Within the host, Mycoplasma bovis bacteremia results in hematogenous spread to other sites and can lead to mastitis, otitis, and arthritis.13,32 Mastitis may be the result of udder-to-udder transmission or spread from other primary sites of infection and can affect dairy cows in all life stages, including dry cows.32 The infection varies from subclinical to severe with multiple quarters affected and results in fibrinosuppurative to caseonecrotic mastitis.
Otitis media due to M. bovis tends to affect dairy and beef calves two to four months of age.27,32 Infection may be unilateral or bilateral, and most affected calves have concurrent M. bovis pneumonia. Initial stages are characterized by ear pain and head shaking. As the disease progresses, animals may become febrile, and involvement of the facial nerve can cause drooping of the ear and ptosis. Severe infections may progress to the inner ear and possible meninges, resulting in dysfunction of the vestibulocochlear nerve and glossopharyngeal nerve.32 Histologically, the otitis is characterized by suppurative inflammation with extensive bony lysis.27
Arthritis due to M. bovis frequently occurs in concert with pneumonia or mastitis and is a feature of chronic pneumonia and polyarthritis syndrome (CPPS) described in feedlot cattle.32 Clinical signs are typical of a septic arthritis, and large high-movement joints such as the hips and stifle tend to be affected. Severe cases are characterized by fibrinosuppurative arthritis with synovial hyperplasia, erosion of articular cartilage, and extension of edema, necrosis, and fibrosis into the periarticular tissues.19,32
References:
- Aebi M, Bodmer M, Frey J, Pilo P. Herd-specific strains of Mycoplasma bovis in outbreaks of mycoplasmal mastitis and pneumonia. Vet Microbiol. 2012;157:363-368.
- Alberti A, Addis MF, Chessa B, et al. Molecular and antigenic characterization of a Mycoplasma bovis strain causing an outbreak of infectious keratoconjunctivitis. J Vet Diagnostic Investig. 2006;18:41-51.
- Arcangioli MA, Duet A, Meyer G, et al. The role of Mycoplasma bovis in bovine respiratory disease outbreaks in veal calf feedlots. Vet J. 2008;177:89-93.
- Ayling R, Nicholas R, Hogg R, et al. Mycoplasma bovis isolated from brain tissue of calves. Vet Rec. 2005;156:391-392.
- Benz R, Piselli C, Potter AA. Channel formation by LktA of Mannheimia (Pasteurella) haemolytica in lipid bilayer membranes and comparison of channel properties with other RTX-Cytolysins. Toxins (Basel). 2019;11:16.
- Biddle MK, Fox LK, Evans MA, Gay CC. Pulsed-field gel electrophoresis patterns of Mycoplasma isolates from various body sites in dairy cattle with Mycoplasma mastitis. J Am Vet Med Assoc. 2005;227:455-459.
- Burgi N, Josi C, Burki S, Schweizer M, Pilo P. Mycoplasma bovis co-infection with bovine viral diarrhea virus in bovine macrophages. Vet Res. 2018;49.
- Burki S, Frey J, Pilo P. Virulence, persistence and dissemination of Mycoplasma bovis. Vol. 179, Veterinary Microbiology. 2015:8.
- Calcutt MJ, Lysnyansky I, Sachse K, Fox LK, Nicholas RAJ, Ayling RD. Gap analysis of Mycoplasma bovis disease, diagnosis and control: An aid to identify future development requirements. Transbound Emerg Dis. 2018;65.
- Caswell JL, Williams WJ. Respiratory System. In: Maxie GM, ed. Vol. 2, Jubb, Kennedy, and Palmer's pathology of domestic animals. St. Louis, Missouri: Elsevier, Inc; 2016:465-591.
- Caswell JL, Archambault M. Mycoplasma bovis pneumonia in cattle. Anim Heal Res Rev. 2007;8:161-186.
- Caswell JL, Bateman KG, Cai HY, Castillo-Alcala F. Mycoplasma bovis in respiratory disease of feedlot cattle. Vol. 26, Veterinary Clinics of North America - Food Animal Practice. 2010:
- Dudek K, Szacawa E. Mycoplasma bovis infections: Occurrence, diagnosis and control,. Vol. 9, Pathogens. 2020:640.
- Dupuy V, Manso-Silvan L, Barbe V, et al. Evolutionary history of contagious bovine pleuropneumonia using next generation sequencing of Mycoplasma mycoides Subsp. mycoides 'Small Colony'. PLoS One. 2012;7:9.
- Fox LK. Prevalence, incidence and risk factors of heifer mastitis. Vet Microbiol. 2009;134:82-88.
- Francoz D, Fecteau G, Desrochers A, Fortin M. Otitis media in dairy calves: A retrospective study of 15 cases (1987 to 2002). Can Vet J. 2004;45:661-666.
- Fujie K, Shinguh Y, Yamazaki A, Hatanaka H, Okamoto M, Okuhara M. Inhibition of elastase-induced acute inflammation and pulmonary emphysema in hamsters by a novel neutrophil elastase inhibitor FR901277. Inflamm Res. 1999;48:160-167.
- Fulton RW, Blood KS, Panciera RJ, et al. Lung pathology and infectious agents in fatal feedlot pneumonias and relationship with mortality, disease onset, and treatments. J Vet Diagnostic Investig. 2009;21:464-477.
- Gagea MI, Bateman KG, Shanahan RA, et al. Naturally occurring Mycoplasma bovis-associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagnostic Investig. 2006;18:29-40.
- Hale HH, Helmboldt CF, Plastridge WN, Stula EF. Bovine mastitis caused by a Mycoplasma species. Cornell Vet. 1962 Oct;52:582-591.
- Hazelton MS, Sheehy PA, Bosward KL, et al. Short communication: Shedding of Mycoplasma bovis and antibody responses in cows recently diagnosed with clinical infection. J Dairy Sci. 2018;101:584-589.
- Hermeyer K, Peters M, Brugmann M, Jacobsen B, Hewicker-Trautwein M. Demonstration of Mycoplasma bovis by immunohistochemistry and in situ hybridization in an aborted bovine fetus and neonatal calf. J Vet Diagnostic Investig. 2012;24:364-369.
- Hirth RS, Nielsen SW, Plastridge WN. Bovine Salpingo-oophoritis Produced with Semen Containing a Mycoplasma. Vet Pathol. 1966;3:616-632.
- Jimbo S, Suleman M, Maina T, Prysliak T, Mulongo M, Perez-Casal J. Effect of Mycoplasma bovis on bovine neutrophils. Vet Immunol Immunopathol. 2017;188:27-33.
- Kanda T, Tanaka S, Suwanruengsri M, et al. Bovine Endocarditis Associated with Mycoplasma bovis. J Comp Pathol. 2019;171.
- Kinde H, Daft BM, Walker RL, Charlton BR, Petty R. Mycoplasma bovis associated with decubital abscesses in Holstein calves. J Vet Diagnostic Investig. 1993;5:194-197.
- Lamm CG, Munson L, Thurmond MC, Barr BC, George LW. Mycoplasma otitis in California calves. J Vet Diagnostic Investig. 2004;16:397-402.
- Ledger L, Eidt J, Cai HY. Identification of antimicrobial resistance-associated genes through whole genome sequencing of mycoplasma bovis isolates with different antimicrobial resistances. Pathogens. 2020;9:588.
- Levisohn S, Garazi S, Gerchman I, Brenner J. Diagnosis of a mixed mycoplasma infection associated with a severe outbreak of bovine pinkeye in young calves. J Vet Diagnostic Investig. 2004;16:579-581.
- L-pez A, Martinson SA. Respiratory System, Mediastinum, and Pleurae. In: Zachary FJ, ed. Pathologic Basis of Veterinary Disease Expert Consult. St. Louis, Missouri: Elsevier Inc.; 2017:471-560.
- Lysnyansky I, Ayling RD. Mycoplasma bovis: Mechanisms of resistance and trends in antimicrobial susceptibility. Front Microbiol. 2016;7.
- Maunsell FP, Woolums AR, Francoz D, et al. Mycoplasma bovis infections in cattle. J Vet Intern Med. 2011;25:772-783.
- Morowitz HJ. When PPLO became mycoplasma. American Scientist. 2011;99:102-105.
- Nicholas RAJ. Bovine mycoplasmosis: Silent and deadly. Vet Rec. 2011;168:459-462.
- Nocard, Roux. The microbe of pleuropneumonia. Clin Infect Dis. 1990;12:354-358.
- Parker AM, Sheehy PA, Hazelton MS, Bosward KL, House JK. A review of mycoplasma diagnostics in cattle. Vol. 32, Journal of Veterinary Internal Medicine. 2018:
- Perez-Casal J. Pathogenesis and Virulence of Mycoplasma bovis. Vet Clin North Am - Food Anim Pract. 2020;36:269-278.
- Prysliak T, Van Der Merwe J, Lawman Z, et al. Respiratory disease caused by Mycoplasma bovis is enhanced by exposure to bovine herpes virus 1 (BHV-1) but not to bovine viral diarrhea virus (BVDV) type 2. Can Vet J. 2011;52.
- Register KB, Olsen SC, Sacco RE, et al. Relative virulence in bison and cattle of bison-associated genotypes of Mycoplasma bovis. Vet Microbiol. 2018;222:55-63.
- Singh K, Ritchey JW, Confer AW. Mannheimia haemolytica: Bacterial-host interactions in bovine Pneumonia. Vet Pathol. 2011;48:338-348.
- Stalheim OHV, Page LA. Naturally occurring and experimentally induced mycoplasmal arthritis of cattle. J Clin Microbiol. 1975;2:165-168.
- Stipkovits L, Ripley P, Varga J, Palfi V. Clinical study of the disease of calves associated with Mycoplasma bovis infection. Acta Vet Hung. 2000;48.
- Thomas A, Ball H, Dizier I, et al. Isolation of Mycoplasma species from the lower respiratory tract of healthy cattle and cattle with respiratory disease in Belgium. Vet Rec. 2002;151:472-476.
- Weisburg WG, Tully JG, Rose DL, et al. A phylogenetic analysis of the mycoplasmas: Basis for their classification. J Bacteriol. 1989;171:6455-6467.
- Wise KS, Calcutt MJ, Foecking MF, et al. Complete genome sequence of Mycoplasma bovis type strain PG45 (ATCC 25523). 79, Infection and Immunity. 2011:982-983.