Signalment:
Gross Description:
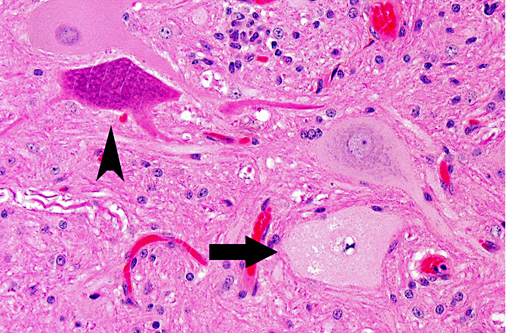
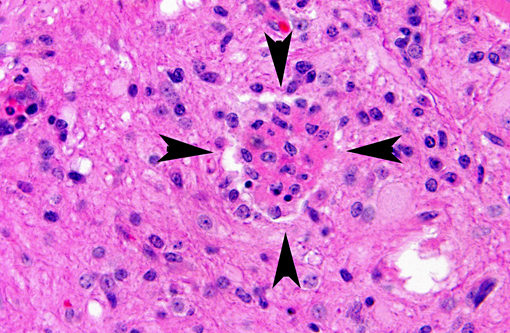
Histopathologic Description:
Brain (not provided): there were similar but milder lesions with minimal neuronal changes in the brain stem; a mild multifocal nonsuppurative leptomeningitis was also present.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
PEVs are ubiquitous and intestinal infection is frequent but most infections are asymptomatic. PEVs undergo local replication in the large intestine and ileum, mucosal lymphoid tissues and lymph nodes, followed by viremia that can lead to the central nervous system infection. The infrequent disease can appear in the postweaning period (generally 6 to 10 week-old pigs) secondary to mixing of pigs from different sources and decreased maternal immunity, and can result in mild to severe neurological disorders.(2) PEV serotype I is highly virulent and the causative agent of Teschen disease. Clinical signs are acute and include fever, ataxia, seizures, convulsions, opisthotonos, coma and death; mortality is high. Milder infections (also known as Talfan disease, poliomyelitis suum, benign enzootic paresis and Ontario encephalomyelitis) are linked to less virulent strains and clinical signs include ataxia, limb paresis, flaccid paraparesis or paraplegia; mortality is usually low and most pigs can recover.Â
There are no gross lesions in enteroviral polioencephalomyelitis. Microscopically, lesion distribution depends on the involved strain, but the cerebrospinal axis from the olfactory bulb to the lumbar cord is consistently involved.(3) As described above, there is progressive lymphocytic perivascular cuffing and infiltration of mononuclear cells (nonsuppurative polioencephalomyelitis) into the neuropil secondary to motor neuron degeneration in the ventral gray column. Neuronophagia and glial nodules, characteristic of CNS viral infections, are key features. Gray matter of the spinal cord and adjacent dorsal root ganglia (ganglioneuritis) are more involved than white matter, and changes in the dorsal horn are milder than in the ventral horn.(6) Other changes may be present, such as vacuolation at the periphery of the soma of neurons. There is relative sparing of the cerebral and cerebellar cortices, contrary to the deep substance of the cerebellum that is consistently and severely involved. Lesions can also be found in the pontine nuclei, medulla, thalamus and periaqueductal gray matter.(5) Leptomeningitis may be patchy and sometimes severe in the cerebellum in the older pigs in which the course is prolonged.(3)
Transmission electron microscopic examination reveals separation of ribosomes from the endoplasmic reticulum and loss of Nissl body clusters that provoke a progressive dilation of the endoplasmic reticulum.(5)
Survival is possible sometimes with sequelae. Extraintestinal infections are relatively transient, whereas the virus persists in the large intestine for several weeks.(2)
In this case, differential diagnoses included Teschen disease, Talfan disease and hemagglutinating encephalomyelitis virus (HEV) infection. To our knowledge, Teschen disease is not known to occur in North America and is associated with high mortality rates. HEV was not isolated and would also have induced higher mortality rates. Porcine reproductive and respiratory syndrome (PRRS) should also be considered in cases with concomitant pulmonary lesions.Â
PEV are specific and are not zoonotic. Although PEV are different from the human enterovirus (poliovirus) that is responsible for acute flaccid paralysis called polio, histological lesions induced by poliovirus are quite similar to those described above.(4)
Other enteroviruses are encountered in swine (Swine Vesicular Disease virus) and in mice (Theilers murine encephalomyelitis virus infection). The virus causing avian encephalomyelitis, formerly classified as an enterovirus, has now been reclassified as a hepatovirus.(1)
JPC Diagnosis:
Conference Comment:
Another rule out that was regarded by conference participants was toxic poliomyelomalacia caused by selenium; however, this would present as a bilaterally symmetric malacia with distribution in the white and gray matter, with loss of neurons and endothelial and glial proliferation (3).Â
This case provides wonderful examples of neuronophagia, neuronal necrosis, and satellitosis, which are characteristic of this and other cases of viral encephalitis. It is difficult to distinguish neurogenic viral etiologies by histology alone, and special diagnostic techniques are usually required.
There was some variation between slides in this case, most notably in the number of spinal nerves, some of which had perivascular inflammatory cells. .Â
References:
2. Derbyshire JB: Enterovirus. In Diseases of swine, 8th ed., pp.145-150. Iowa State University Press, Ames, IA; 1999.
3. Maxie MG, Youssef S: Nervous System. In Jubb, Kennedy, and Palmers pathology of domestic animals, Vol. 1, 5th ed., pp.281-457. Elsevier Saunders, Philadelphia, PA, 2007.
4. McAdam AJ, Sharpe AH: Infectious Diseases. In Robbins and Cotran Pathologic Basis of Disease, 8th ed.pp. 331-398. Elsevier Saunders, Philadelphia, PA, 2010.
5. Summers BA, Cummings JF, DeLahunta A: Veterinary neuropathology. 1st ed., pp.123-125. Mosby, St-Louis, MO; 1995.
6. Yamada M, Kozakura R, Nakamura K, Yamamoto Y, Yoshii M, Kaku Y, Miyazaki A, Tsunemitsu H, Narita M. Pathological changes in pigs experimentally infected with porcine teschovirus. J. Comp. Pathol. 2009;141(4):223-228.
7. Zell R, Dauber M, Krumbholz A, Henke A, Birch-Hirschfeld E, Stelzner A, Prager D, Wurm R. Porcine Teschoviruses Comprise at Least Eleven Distinct Serotypes: Molecular and Evolutionary Aspects. J. Virol. 2001;75(4):1620-1631.
8. Zachary JF. Nervous system, mediastinum, and pleurae. In: McGavin MD, Zachary JF, eds., Pathologic Basis of Veterinary Disease. 5th ed., St. Louis, MO:Mosby; 2011:853.