Signalment:
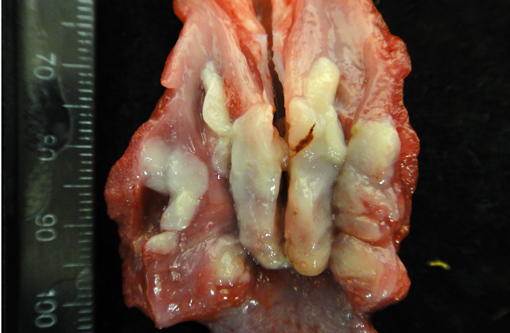
Gross Description:
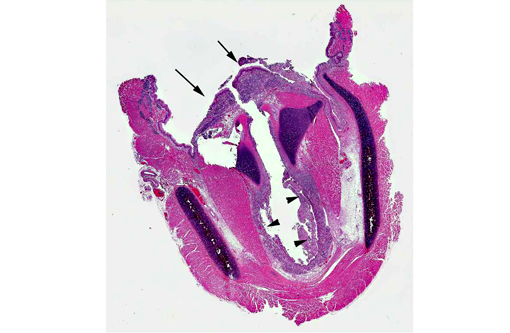
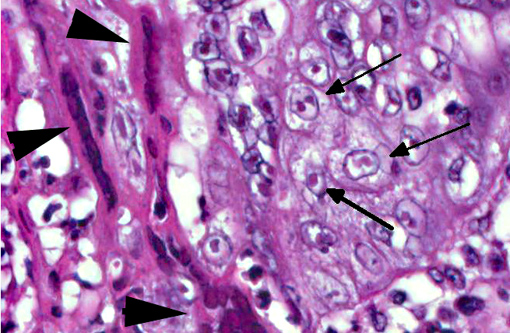
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Aerobic culture, larynx: moderate coagulase negative Staphylococcus spp., moderate Pasteurella multocida
Anaerobic culture, larynx: Few Prevotella spp.
Condition:
Contributor Comment:
Investigations into this virus performed by Galeota et al.(1), including biological behavior and molecular characteristics, supported the inclusion of hyrax herpesvirus in the Alphaherpesvirinae subfamily and Simplexvirus genus. At our institutions we have seen oral ulcers on numerous occasions in rock hyraxes (often as incidental lesions), and previous molecular investigations from individuals in this colony have shown the offending organism to have 100% shared identity with published hyrax herpesvirus DNA polymerase gene sequences.Â
As with multiple other members of the Alphaherpesvirinae, lesions associated with the hyrax herpesvirus are the result of epithelial necrosis and ulceration with secondary inflammation and, in some cases, bacterial infection. Systemic lesions associated with this virus have not been confirmed, but one published case was described as having nonsuppurative meningoencephalitis1 and we have seen neuronal necrosis in one of our cases. As with other herpes viruses, it is suspected that the hyrax herpesvirus causes long-term infection with occasional episodes of recrudescence. As no new animals were recently introduced into this group prior to the death of these two animals, recrudescence was suspected in these cases. The colony had recently been transferred to their indoor winter housing which likely introduced stressors and may have induced shedding of the virus in previously infected animals and overt lesions in these younger individuals.
JPC Diagnosis:
Conference Comment:
Alphaherpesviruses tend to lyse host cells and typically result in widespread or localized necrosis, as in this case. They grow rapidly, producing Cowdry type-A intranuclear inclusion bodies (and viral syncytial cells) and establishing lifelong latent infections in both the lymphoreticular system and the trigeminal ganglion.(4) Gallid herpesvirus 2 (Mareks disease) is a rare example of an alphaherpesvirus which acts more like a gammaherpesvirus in that it induces a lymphoproliferative response.(2) Table 1 summarizes other alphaherpesviruses of veterinary importance.(2,4)
Directional spread of alphaherpesviruses within the nervous system and the establishment of latency is a critical component of the viral lifecycle. The virus initially replicates peripherally in the skin or mucus membranes, where the innate immune response provides the first line of defense. Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns (PAMPs) expressed by the virus. Activated TLRs result in the production of cytokines, which recruit macrophages and/or induce proteins that degrade mRNA and inhibit translation. The adaptive immune response also works to prevent viral spread via viral specific CD8+ cytotoxic T-cells.(3) Although the initial infection is typically cleared within a few weeks, the virus spreads along axons to the sensory nerve ganglion, where the viral genome is maintained indefinitely. During latency, normally productive viral genes are quiescent and unproductive and LATs (latency associated RNA transcripts) accumulate in neuronal nuclei. LATs are Bcl-2 analogs (Bcl-2 is an anti-apoptotic regulator protein) that confers resistance to apoptosis, allowing viral persistence in sensory neurons.(5) Thus, in times of stress or immune suppression, the virus can reactivate and cause clinical disease.
Table 1: Alphaherpesviruses of veterinary importance.2,4
| Bovine herpesvirus 1 | Infectious bovine rhinotracheitis, infectious pustular vulvovaginitis |
| Bovine herpesvirus 2 | Bovine mammillitis/pseudo-lumpy skin disease |
| Bovine herpesvirus 5 | Bovine herpes meningoencephalitis |
| Porcine herpesvirus 1 | Pseudorabies/Aujeszkys Disease |
| Equine herpesvirus 1 | Equine abortion |
| Equine herpesvirus 3 | Equine coital exanthema |
| Equine herpesvirus 4 | Equine rhinopneumonitis |
| Equine herpesvirus 5 | Multinodular pulmonary fibrosis |
| Gallid herpesvirus 1 | Avian infectious laryngotracheitis |
| Gallid herpesvirus 2 | Mareks disease |
| Psittacid herpesvirus | Pachecos disease |
| Anatid herpesvirus 1 | Duck Plague |
| Feline herpesvirus 1 | Feline viral rhinotracheitis |
| Canine herpesvirus 1 | Canine herpesviral disease |
| Macacine herpesvirus 1 | B-virus of macaques |
| Saimiriine herpesvirus 1 | Herpes tamarinus |
References:
2. MacLachlan NJ, Dubovi EJ eds. Fenners Veterinary Virology. 4th ed. London, UK; 2011:179-201.
3. Kramer T, Enquist LW. Directional spread of alphherpesvirus in the nervous system. Viruses. 2013;5:678-707.
4. Zachary JF. Mechanisms of microbial infections. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby; 2012:212-238.
5. Zerboni L, Che X, Reichelt M, Qiao Y, Gu H, Arvin A. Herpes simplex virus 1 tropism for human sensory ganglion neurons in the severe combined immunodeficiency mouse model neuropathogenesis. J Virol. 2013;87(5):2791-2802.