CASE 2: 14A030 (4048445-00)
Signalment:
5.74-years-old, female Indian rhesus macaque (Macaca mulatta)
History:
This animal had chronic diarrhea. Three days prior to the necropsy, this animal presented with hypothermia, hypoglycemia, and dehydration. There was not improvement in spite of antibiotic treatment. Euthanasia was elected due to the poor prognosis.
Gross Pathology:
The animal showed thin body condition and muscular atrophy. Both eyes were sunken. The pancreas was pale and edematous. Diffusely the small intestine, cecum and colon were extremely dilated with large amount of fluid, blood-tinged fecal material. The mesenteric lymph nodes were edematous and enlarged.
Laboratory results:
Serum or EDTA blood testing for HVP2, MEASLES, SIV, SRV, STLV1, SRV2 were negative by MFIA and PCR. Yersinia enterocolitica were isolated from the colon 3 days prior to the necropsy.
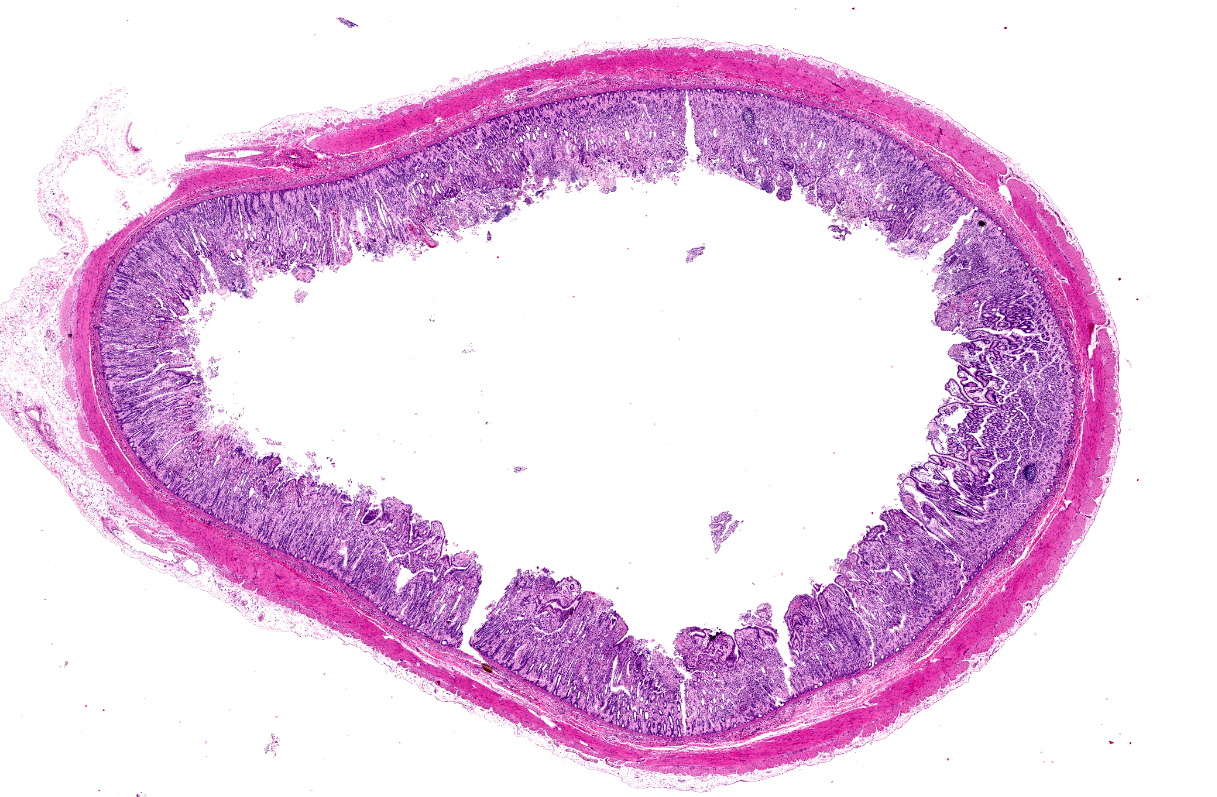
Microscopic description:
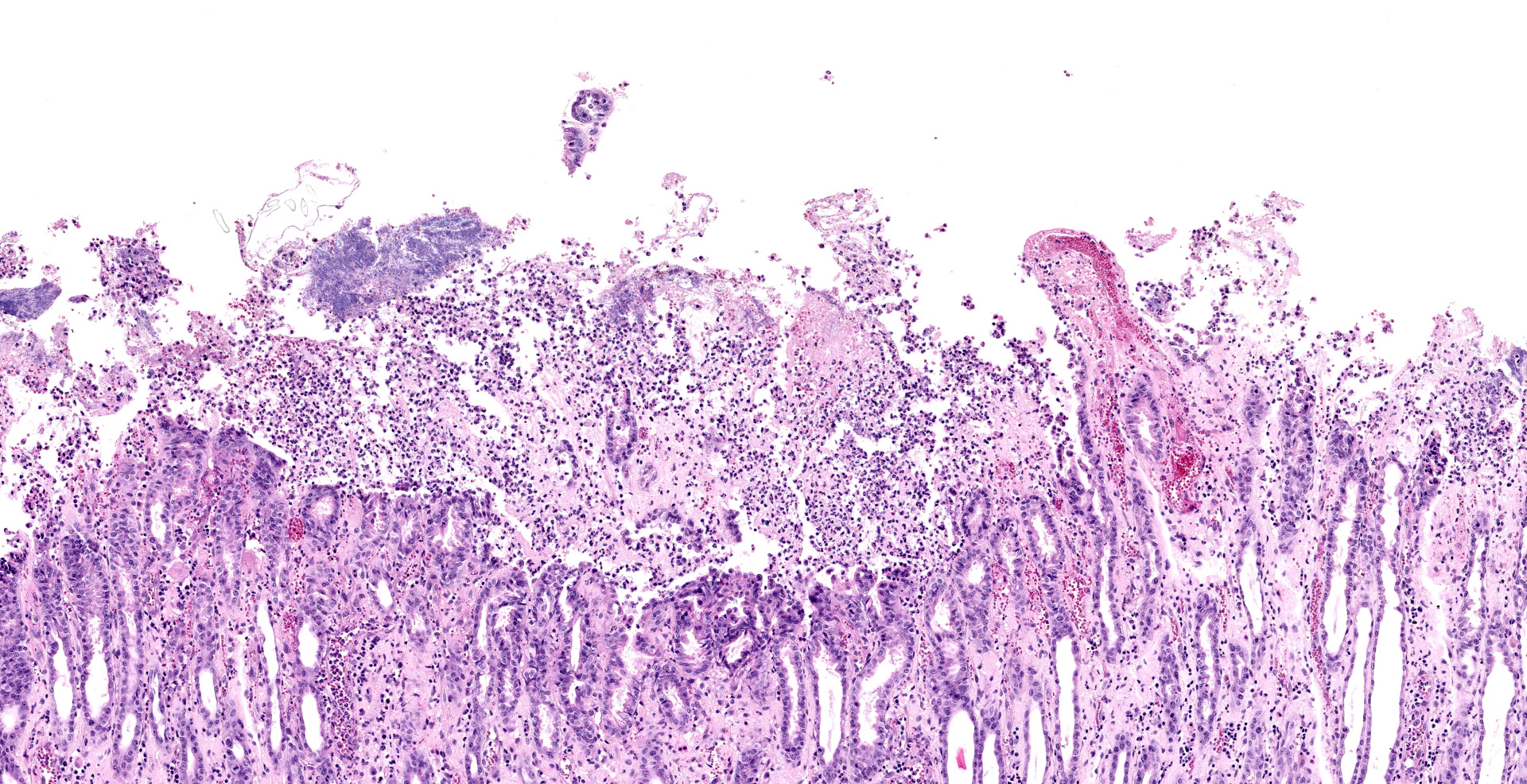
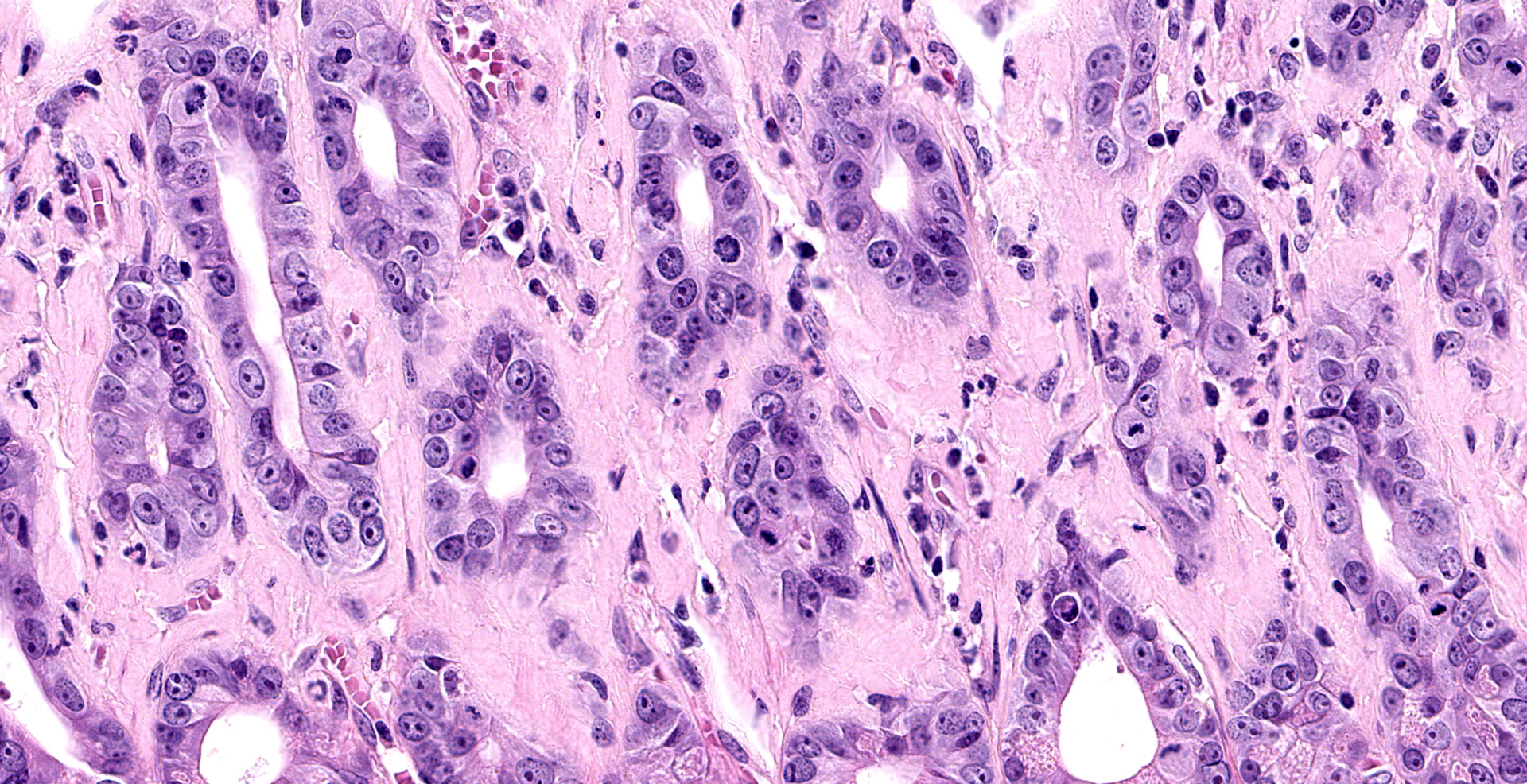
Jejunum. The mucosa has multifocal to coalescing, mild to moderate erosions and/or ulcerations and replaced by large amount of eosinophilic cellular debris, degenerate neutrophils, fibrin and erythrocytes (hemorrhage). Admixed there are numerous colonies of 1 x 2 um bacilli (Gram negative). Diffusely the submucosa and serosa are mildly to moderately expanded by edema with many inflammatory infiltrates. Multifocally the blood and lymphatic vessels are dilated and surrounded by edema and many inflammatory infiltrates (perivasculitis). Occasionally the tunica media of vessels is expanded and disrupted by inflammatory cells (vasculitis). Diffusely the lamina propria is expanded and contains abundant amorphous, finely fibrillar to waxy hyalinized deposits (Congo red stain positive).
Contributor's morphologic diagnosis:
1. Small intestine: Enteritis, acute, diffuse, necrohemorrhagic, with perivasculitis, vasculitis, and colonies of bacilli, etiology consistent with Yersinia enterocolitica, Indian rhesus macaque (Macaca mulatta).
2. Small intestine: amyloidosis, diffuse, moderate, Indian rhesus macaque (Macaca mulatta).
Contributor's comment:
Yersinia is a member of Enterobacteriaceae, which encompasses 17 species including three important pathogenic species: Y. enterocolitica, Y. pseudotuberculosis and Y. pestis. Y. enterocolitica is a globally distributed gastrointestinal pathogen and usually causes a self-limiting acute infection beginning in the intestine and spreading to the mesenteric lymph nodes.7 However, it can cause serious or chronic infections, particularly in immunocompromised individuals. The pathogenesis of Y. enterocolitica is not completely clear. Y. enterocolitica contains more than 16 virulence-associated genes. The plasmid gene of pYV/pCD and chromosomal genes of ystA, ystB, and ystC are the most important for the pathogenesis. pYV/pCD gene functions to allow bacterium to penetrate the intestinal wall. ystA, ystB, and ystC are the stable heat-stable toxin genes that may contribute to the pathogenesis of diarrhea.8
Y. enterocolitica infects many species of NHPs and causes outbreaks of diarrhea. Fatal yersiniosis has been documented in captive squirrel monkeys and gibbons. The major lesions are necrotizing enteritis and abscesses in many other organs.7 The differential diagnosis in nonhuman primates includes Shigella, Campylobacter, Salmonella and E. coli.
Reactive amyloidosis is not an uncommon finding in rhesus in our colonies. The major organs involved are intestine, mesenteric lymph nodes, spleen, and liver, but less often in kidney and other organs. Reactive amyloidosis is caused by the deposition of serum amyloid, which is a circulating acute-phase reactant produced mainly by hepatocytes, and regulated by interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF). Reactive amyloidosis has been reported in human patients with rheumatoid arthritis and other chronic inflammatory diseases.9 Y. pseudotuberculosis has been reported to be the cause of amyloidosis in humans,1 but the association of Y. enterocolitica and entero-amyloidosis in this case is unclear.
Contributing Institution:
Department of Comparative Pathology
Tulane National Primate Research Center
JPC diagnosis:
1. Small intestine: Enteritis, necrosuppurative, multifocal, moderate, with large colonies of bacilli.
2. Jejunum, mucosa: Amyloidosis, diffuse, moderate.
JPC comment:
Pigs are generally considered the reservoir host of Yersinia enterocolitica, and is a widespread pathogen across Europe and Poland. While many cases continue to be reported, detection and identification of this bacterium remain difficult. It has recently been shown that diagnostics can be improved with the use of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI TOF MS), but at the cost of time and additional media and equipment preparation.6
There are a number of virulence factors that aid Y. enterocolitica in establishing infection in tissues. Some of the most well characterized include the three adhesins/invasins Inv, YadA, and Ail, which facilitate attachment to host cells, and confer the ability to survive neutrophils and in Peyer's patches. Before attaching to target host cells, this bacterium has a flagella and motility increases its virulence. Either the flhDC or fliA gene must be functional to maintain a functional flagellum, and experimental inactivation results in decreased virulence in vitro. Lipopolysaccharide of the outer membrane is a potent virulence factor that is shared by Gram negative bacteria.4
However, the most important virulence factor is the Yop virulon, which elaborates its effectors to the extracellular space, the plasma membrane, and the host cell cytosol, and the Ysc type 3 secretion system (T3SS). The T3SS consists of the injectisome and the translocators YopB, YopD, and LcrV, and the T3SS counteracts multiple innate defenses of phagocytes by inhibiting cytoskeleton movement and preventing its phagocytosis.4
While more strictly public health facing, there has been research into the use of bacteriophages to limit human exposure and consumption of food contaminated by Yersinia enterocolitica. Viruses of Podoviridae and Myoviridae were shown to be safe in mice, and several phages significantly reduced the population of experimentally placed Y. enterocolitica on kitchen utensils and food. The bacteria exhibit different morphologic characteristics based on temperature, so whether this represents a potential in vivo therapy will require additional research.5
There is widespread concern about the prudent use of antibiotics in the treatment of susceptible disease, and research regarding alternative therapies against Y. enterocolitica continue. Aporphinoids are a subgroup of benzylisoquinoline alkaloid compounds found in plants, and several demonstrated significant antibacterial properties against Y. enterocolitica (as well as some efficacy against a Salmonella enterica strain, E. coli strain, and Staphylococcus aureus strain).3 Additionally, colicin FY, which is typically produced by Yersinia frederiksenii, has demonstrated efficacy against Y. enterocolitica in mouse models. Murine E. coli isolates were transformed with a colicinogenic plasmid in order to induce production of colicin FY and tested in vitro and in vivo with excellent results.2 Whether either of these represent potential future therapies will be determined by future research.
References:
1. Bevanger, L. Yersinia pseudotuberculosis as the cause of septicaemia in a patient with amyloidosis. Acta Pathol. Microbiol. Scand. 1976; 84:461?462.
2. Bosak J, Micenkova L, Hrala M, et al. Colicin FY inhibits pathogenic Yersinia enterocolitica in mice. Nature. 2018;8:12242.
3. Di Marco N, Lucero-Estrada C, Pungitore CR. Aporphinoid alkaloids as antimicrobial agents against Yersinia enterocolitica. Letters in Applied Microbiology.2019;68:437-445.
4. Fabrega A, Vila J. Yersinia enterocolitica: Pathogenesis, virulence and antimicrobial resistance. Enferm. Infecc. Microbiol Clin. 2012;30(1):24-32.
5. Jun JW, Park SC, Wicklund A, Skurnik M. Bacteriophages reduce Yersinia enterocolitica contamination of food and kitchenware. International Journal of Food Microbiology. 2018;20(271):33-47.
6. Morka K, Bystron J, Bania J, et al. Identification of Yersinia enterocolitica isolates from humans, pigs and wild boars by MALDI TOF MS. BMC Microbiology. 2018;18(1):86.
7. Nakamura, S., Hayashidani, H., Iwata, T., Namai, S. & Une, Y. Pathological Changes in Captive Monkeys with Spontaneous Yersiniosis due to Infection by Yersinia enterocolitica serovar O8. J. Comp. Pathol. 2010; 143:150?156.
8. Sabina, Y., Rahman, A., Ray, R. C. & Montet, D. Yersinia enterocolitica: Mode of Transmission, Molecular Insights of Virulence, and Pathogenesis of Infection. J. Pathog. 2011, 1?10 (2011).
9. Sponarova, J., Nyström, S. N. & Westermark, G. T. AA-Amyloidosis Can Be Transferred by Peripheral Blood Monocytes. 2008; PLoS ONE 3, e3308.