Signalment:
Juvenile, undetermined sex, bluegill fish (
Lepomis macrochirus)A group of juvenile bluegill was purchased from a commercial fish hatchery for use in respiratory physiology studies. The fish were then group-housed in a large indoor water tank. Approximately 2 weeks after their arrival in the laboratory, several fish were noted to have white cutaneous nodules on their fins and/or bodies. Three affected fish were removed from the tank, euthanized, and submitted for necropsy.
Gross Description:
A 3 mm diameter, firm, white, multilobulated, exophytic, nodular, cutaneous mass protrudes from the dorsal midline of fish #1 at the cranial margin of the dorsal fin (
Fig. 3-1). Three smaller (<1mm) white nodules are present along the dorsocaudal aspect of this fishs dorsal fin. Similar nodules of varying sizes and lobulation are located on the caudal peduncle, anal fin, and ventral midline of fish #2 and the tail fin and ventral midline of fish #3.Â
Histopathologic Description:
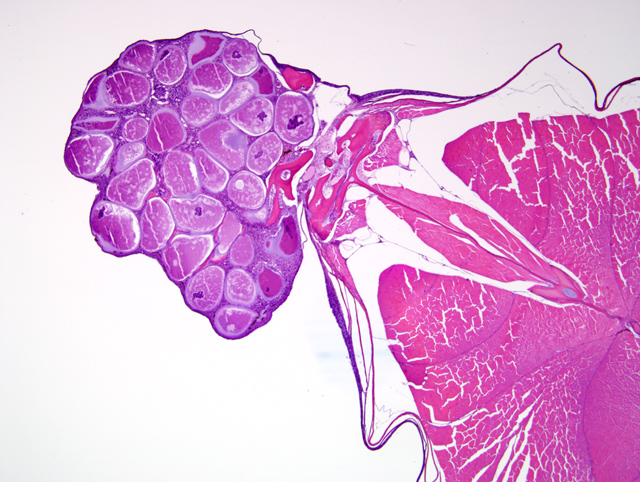
A tranverse section of the whole body is present on the slide. The skin overlying the dorsal midline and, in some slides, including the base of the dorsal fin, contains an irregularly shaped nodular dermal mass measuring up to 2 mm in greatest diameter (
Fig. 3-2). This mass contains multiple large round to oval cells (
Fig. 3-3), measuring up to 350 μm in diameter, each of which has a 2-10 μm thick lightly basophilic hyaline capsule, abundant amounts of flocculent basophilic cytoplasm, and often a single irregularly shaped degenerate karyomegalic nucleus (up to 80 μm in diameter) containing condensed chromatin and a single large nucleolus. Within the cytoplasm of most of these cells, primarily in a subcapsular location, are irregularly shaped basophilic inclusions that often coalesce to form a fine lattice. Multifocally, there is rupture of the cell capsule and/or intracellular infiltration by macrophages and lymphocytes. The dermis between the large cells is diffusely infiltrated by low to moderate numbers of macrophages and lymphocytes. Multifocally, the overlying epidermis is mildly hyperplastic. However, there are also foci of epidermal erosion.Â
No significant changes are present in other organs of this fish.Â
Morphologic Diagnosis:
Skin; focal (nodular) dermal fibroblast hypertrophy, severe, with karyomegaly, hyaline capsule formation, basophilic intracytoplasmic inclusions, degeneration, lymphohistocytic inflammation, epidermal hyperplasia, and epidermal erosion
Condition:
Lymphocystis
Contributor Comment:
All histology slides submitted for this case are from fish #1, and the lesion present is depicted grossly in Figure 3-1. The gross and histologic findings are consistent with the fish disease known as lymphocystis.(5) The severely hypertrophied dermal fibroblasts with hyaline capsules and basophilic intracytoplasmic inclusions are pathognomonic for this disease and are known as lymphocystis cells; an individual cell, which may enlarge up to a million (106) times normal size, appears grossly as a cream-colored nodule or lymphocyst measuring up to 2 mm in diameter.(1) Nodules may occur singly or grouped together in raspberry-like clusters (as in this lesion). These nodules are most commonly seen on the fins and body. Occasionally, the gills, peritoneum, and/or internal organs may be affected.(1,5)
Lymphocystis is a common and widespread disease of teleost fish that has been reported in more than 140 species of freshwater and marine fish.(1) Species in the order Perciformes (which includes bluegill) and order Pleuronectiformes (flatfish such as flounder, sole, plaice, and dab) constitute approximately 75% and 10%, respectively, of the species affected.(1)
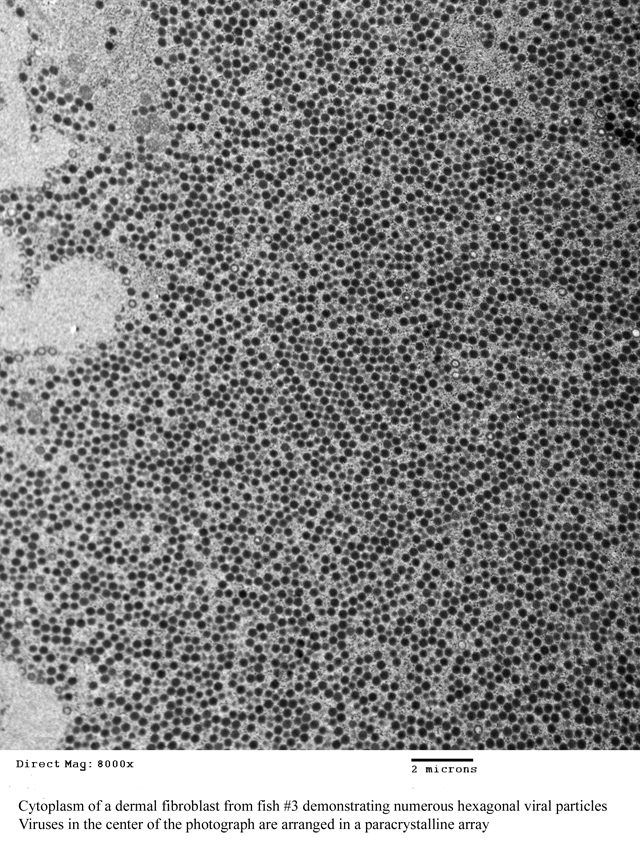
This disease is caused by infection with lymphocystis disease virus (LDV), which is a DNA virus in the genus Lymphocystivirus of the family
Iridoviridae.(3) Currently, there are two recognized species of LDV: LDV-1 and LDV-2.(3) However, there appear to be different strains of the viruses that vary in size and host specificity.(1,5) Viral particles are non-enveloped and display icosahedral symmetry; by transmission electron microscopy (TEM), they usually appear hexagonal and may form paracrystalline arrays within the cytoplasm of infected cells as seen in one of the lesions removed from fish #3 (
Fig. 3-4).
Viral infection may occur through the gills, digestive tract, or skin wounds.(1) Infected fibroblasts soon begin to enlarge; increases in cytoplasm are accompanied by nuclear and nucleolar enlargement.(5) As the cell enlarges, irregularly shaped basophilic cytoplasmic inclusions develop; TEM reveals that these are sites of viral assembly.(1) The characteristic hyaline capsule of a lymphocystis cell appears at approximately the mid-stage of maturation.(5) Cellular degeneration begins with nuclear condensation followed by breakdown of the capsule; infiltrates of macrophages and lymphocytes surround and eventually invade degenerate cells.(5) Rupture of the degenerate cells releases the viral particles which may then infect adjacent fibroblasts or, if released into the environment, infect other fish.(1,5)
Lymphocystis is a benign disease with very low mortality; lesions usually heal completely. However, secondary bacterial or fungal infection of the lesions may result in debilitation and/or death.(1)
Interestingly, the first isolation and in vitro cultivation of LDV was done with samples collected from infected bluegill and the first experimental pathogenesis studies were performed with this fish species.(2) In these studies it was found that for fish kept at 25oC the time from experimental infection to lesion regression was approximately 28 days.(2) In a later study with experimentally infected plaice kept at 10oC, it took 3 months for the lesions to regress.(5)
After the diagnosis of lymphocystis in the three bluegill described above, the following measures were taken to control the disease in the remaining group of fish: 1) the fish were closely examined and each one with visible lymphocystis lesions was removed and euthanized; 2) water flow through the holding tank was increased to flush out any viral particles in the tank; and 3) the temperature of the water in the tank was increased to 25oC to enhance the immune function of the fish and inhibit virus survival in the tank environment. These control measures were successful; no additional cases of lymphocystis have since been identified in these fish.
JPC Diagnosis:
Scaled skin: Fibroblast hypertrophy, nodular, focal with karyomegaly, basophilic cytoplasmic inclusions and moderate lymphoplasmacytic dermatitis
Conference Comment:
The contributor did an outstanding job of covering all the salient features of lymphocystis. Viruses in the family
Iridoviridae generally affect fish, amphibians and invertebrates. Within the family there are 5 genera: 1)
Iridovirus (the iridoviruses); 2)
Chloriridovirus (large iridescent insect viruses); 3)
Ranavirus (frog iridoviruses); 4)
Lymphocystivirus (lymphocystis viruses of fish); and 5) unnamed (goldfish iridoviruses). African swine fever was once considered part of the
Iridoviridae family, but it has recently been recategorized into its own family.
References:
1. Anders K: Lymphocystis disease of fishes.Â
In: Viruses of Lower Vertebrates, eds. Ahne W, Kurstak E, pp. 141-160. Springer-Verlag, New York, NY, 1989
2. Dunbar CE, Wolf K: The cytological course of experimental lymphocystis in the bluegill. J Infect Dis
116:466-472, 1966
3. ICTVdB Management (2006). 00.036. Iridoviridae.Â
In:
ICTVdB - The Universal Virus Database, version 3, B+�-+chen-Osmond, C. (Ed), Columbia University, New York, NY http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/
4. Murphy, FA: Virus taxonomy.Â
In: Fundamental Virology, eds. Fields BN, Knipe DM, Howley PM, 3rd ed., pp. 47-48. LippincottRaven Publishers, Philadelphia, PA, 1996
5. Roberts RJ: The virology of teleosts.Â
In: Fish Pathology, 2nd ed., pp. 192-195. Bailliere Tindall, London, UK, 1989