Signalment:
Gross Description:
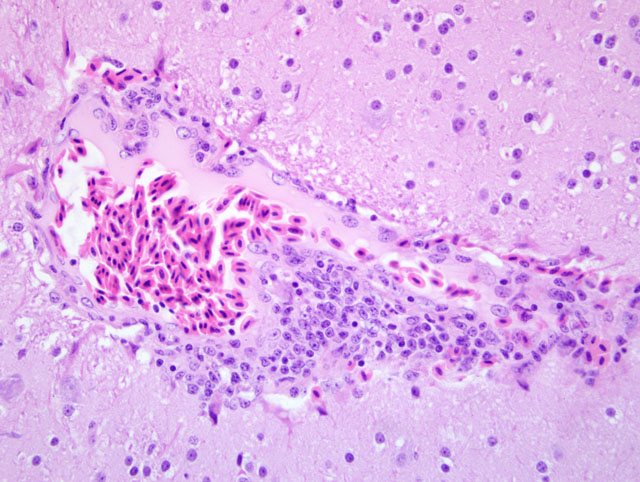
Histopathologic Description:
Crop: Variable numbers of plasma cells and lymphocytes infiltrate around and within blood vessels, nerves and ganglia in the tunica muscularis. Infrequently, a few plasma cells and lymphocytes have infiltrated between smooth muscle fibers of the tunica muscularis. Occasional moderate sized lymphoid aggregates are present in the lamina propria.
Morphologic Diagnosis:
1. Mild to moderate cerebral and meningeal lymphoplasmacytic perivascular cuffing.
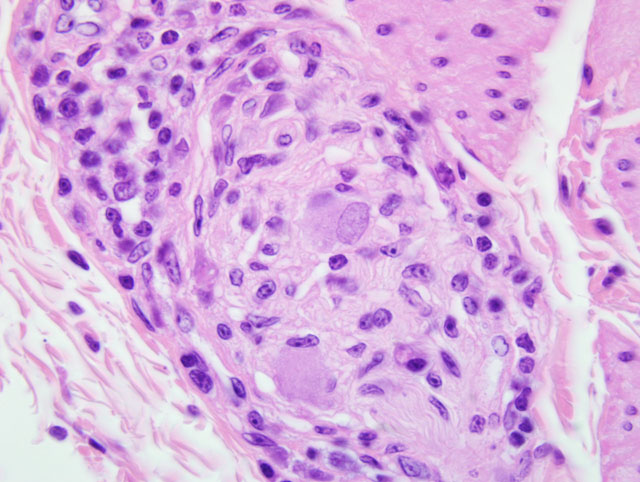
2. Mild to moderate multifocal lymphoplasmacytic ganglioneuritis and mild multifocal non-suppurative leiomyositis of the crop compatible with proventricular dilatation disease (PDD)
Lab Results:
Condition:
Contributor Comment:
The characteristic cellular infiltrates of PDD are variable in size and distribution as demonstrated by the lesions in the sections of brain and crop provided for this case. In some sections of crop, numerous plasma cells and lymphocytes are present within the nerves and ganglia, while in other sections, only rare plasma cells or lymphocytes can be identified. Historically, examination of multiple sections of biopsied tissues, such as crop, or multiple sections of gastrointestinal tract collected at necropsy was needed to improve diagnostic accuracy.
An avian bornavirus (ABV) has recently been proposed as the etiological agent of PDD, and evidence from bird inoculation studies is strongly supportive.(4,5) Through collaborative efforts of researchers from the University of California, San Francisco, the Ontario Veterinary College and the Animal Health Laboratory, University of Guelph, an ABV specific RT-PCR screening test and immunohistochemistry (IHC) that detects the ABV nucleocapsid protein have been developed and tissues from psittacine birds with and without PDD were tested. Those results were compared with the histopathologic diagnoses and the sensitivity and specificity of IHC for detection of ABV antigens on a bird by bird basis were found to be 100% and 100%, respectively. Many more tissues were positive for ABV RNA by RT-PCR than were positive histopathologically or for viral antigens by IHC. Brain tissues, but not crop tissues, from this bird were positive by RT-PCR for ABV antigen. Similar results were obtained with IHC testing. Overall, brain tissue appears to be the tissue that is most consistently positive with both PCR and IHC testing. It is suggested that IHC and or RT-PCR testing of biopsy specimens, in addition to histopathology, may help increase the sensitivity of diagnosis of PDD allowing for earlier identification of infected birds.(11)
JPC Diagnosis:
1. Brain: Meningoencephalitis, lymphohistiocytic and plasmacytic, perivascular, multifocal, mild.
2. Crop, ganglia: Ganglioneuritis, lymphohistiocytic and plasmacytic, multifocal, mild.
Conference Comment:
The pathogenesis and persistence of the virus within infected cells is complex. Once infection is established, the virus interferes with many intracellular signaling pathways involved in spread of the virus, maintenance of viral persistence, and modulation of neurotransmitter pathways.(9) Neuronal death is due to effector CD8+ T cells which kill infected cells.(12) Studies in laboratory infected mice demonstrated a positive correlation between lesion severity and increased levels of IL-6, TNF-α, IL-α, and inducible nitric oxide synthase mRNA.(12)
References:
2. Daoust PY, Julian R, Yason CV, Artsob H. Proventricular impaction associated with nonsuppurative encephalomyelitis and ganglioneuritis in two Canada geese. J Wildlife Dis. 1991; 27:513-517.
3. Degernes LA, Flammer K, Fisher P. Proventricular dilatation syndrome in a Green-Winged Macaw. Proceedings of the Annual Conference of the Association of Avian Veterinarians. 1991;45-49.
4. Gancz AY, Kistler AL, Greninger AL, et al. Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4. Virol J. 2009;6:100.
5. Gray P, Hoppes S, Suchodolski P, et al. Use of avian bornavirus isolates to induce proventricular dilatation disease in conures. Emerg Infect Dis. 2010;16:473-479.
6. Joyner KL, Kock N, Styles D. Encephalitis, proventricular and ventricular myositis, and myenteric ganglioneuritis in an Umbrella Cockatoo. Avian Dis. 1989;33:379-381.
7. Mannl A, Gerlach H, Leipold R. Neuropathic gastric dilatation in Psittaciformes. Avian Dis. 1987;31:214-221.
8. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ. Bornaviridae. In: Veterinary Virology. 3rd ed. vol.1. San Diego, CA: Elsevier Academic Press; 1999:455-458.
9. Planz O, Pleschka S, Wolff T. Borna disease virus: a unique pathogen and its interaction with intracellular signaling pathways. Cell Microbiol. 2009;11(6):872-879.
10.Perpi+â-¦+â-ín D, Fern+â-índez-Bellon H, L³pez C, Ramis A. Lymphoplasmacytic myenteric, subepicardial and pulmonary ganglioneuritis in four non-psittacine birds. J Avian Med Surg. 2007;21:210-214.
11.Raghav R, Taylor M, Delay J, Kistler A, Smith D. Avian bornavirus is present in many tissues of psittacine birds with histopathologic evidence of proventricular dilatation disease. J Vet Diagn Invest. 2010:22(4):495-508.
12.Thakur R, Sarma S, Sharma B. Role of Borna disease virus in neuropsychiatric illnesses: are we inching closer?. Indian J Med Microbiol. 2009;27(3):191-201.