Signalment:
Gross Description:
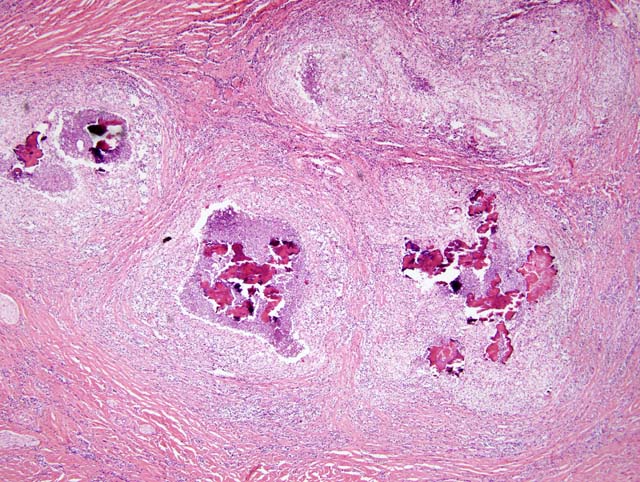
Histopathologic Description:
Grams stain of the tongue: There are moderate numbers of Gram negative cocco-bacilli within the Splendore-Hoeppli material.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Clinically, affected animals present with weight loss and hypersalivation. This is due to the extensive destruction of the tongue and oral tissues. Gross lesions commonly manifest as variably sized, hard, circumscribed nodules that measure up to several millimeters in diameter. These nodules are most commonly found in the tissues and skin of the face, and can progress to form soft abscesses which can fistulate and discharge through the mouth or skin. The discharged purulent material is odorless and contains abundant granules.(6) This infection induces in the tongue a severe fibroblastic response causing it to become large and immobile making chewing and swallowing difficult. This hardening of the tongue is the genesis of the common name of the disease wooden tongue. The formation of granulation tissue within the fibrous connective tissue gives the nodular appearance to these lesions. Additionally, small yellow foci (sulfur granules) can often be seen within the dense granulation tissue. These represent the bacterial colonies within the lesion.
Oral infection with A. lignieresii will commonly spread via lymphatics to local lymph nodes. Infections are less commonly found in the forestomachs, lungs, skin and uterus.(4) Grossly, infected lymph nodes contain yellow-orange granulomatous nodules which frequently project above the normal nodal capsular contour and are surrounded by sclerosing inflammation.(2) Affected lymphatics are diffusely thickened with similar nodules.(2) The most commonly affected lymph nodes are the retropharyngeal and submaxillary nodes.
Histologically, these nodular lesions consist of multiple pyogranulomas surrounding aggregates of Gram negative cocco-bacilli embedded in homogenous eosinophilic material. The hypereosinophilic material along with the bacteria forms the club-shaped microcolonies which is characteristic of this disease.(6) These formations are thought to be associated with immune complex deposition.(2) Surrounding the central area of bacteria is a band of inflammatory cells composed predominantly of neutrophils with macrophages and occasional giant cells. The external layer of the pyogranuloma consists of a dense layer of fibrous connective tissue containing variable numbers of lymphocytes and plasma cells. These lesions are often concurrently infected with Actinomyces pyogenes, Streptococcus spp., and Pseudomonas aeruginosa.(4)
A. lignieresii has been isolated from laboratory rodents associated with middle ear infections and conjunctivitis (5) and from healthy and diseased horses.(3) There is one report of the bacteria being isolated from a horse with an enlarged tongue.(1) In swine it is seen as granulomas or abscesses of the teats associated with wounds from the sharp teeth of suckling piglets. In horses this bacteria infrequently causes lower airway disease and abscess.(4)
The most important differential diagnoses for actinobacillosis include Actinomyces bovis, Staphylococcus aureus (botryomycosis) and Mycobacterium bovis. A. bovis also contains sulfur granules within discharge. However, infections with this agent tend to be associated with infection of bone, in particular the mandible.(4) In addition, A. bovis is a Gram positive filamentous organism, and this infection does not consistently spread to local lymph nodes. Botryomycosis classically has chronic granulomas with centers of numerous Gram positive cocci embedded in a homogenous matrix and the purulent material associated with this infection has botryomycotic granules.(4)
JPC Diagnosis:
Conference Comment:
Splendore-Hoeppli material is intensely eosinophilic, club-shaped material that radiates around certain fungi, bacteria, parasites and biologically inert substances such as suture. The material is generally composed of antigen-antibody complexes, debris and fibrin.(7) The most common infections and conditions that result in the Splendore-Hoeppli phenomenon include botryomycosis, Nocardia sp., Actinomyces sp., and feline dermatophytic pseudomycetomas.(7) It has also been reported with Pythium insidiosum, Sporothrix schenckii, Candida albicans, Aspergillus sp., and zygomycetes such as Conidiobolus sp. and Basidiobolus sp., Coccidioides immitis, nematodes, schistosomiasis, and hypereosinophilic syndrome.(7)
The presence of the Splendore-Hoeppli material led to a discussion of antigen-antibody complexes. Deposition of immune complexes causes tissue damage primarily by activation of the complement cascade and activation of neutrophils and macrophages through their Fc receptors.(8) Immune complexes also cause platelet aggregation and activation of Hageman factor which lead to microthombi formation and kinin activation. Activated complement produces chemotactic factors such as C5a which recruits macrophages and neutrophils, and anaphylatoxins such as C3a and C5a which increase vascular permeability.(8) The leukocytes are activated by binding of their C3b and Fc receptors by the immune complexes. Activated leukocytes release prostaglandins, chemotactic substances, oxygen free radicals, and lysosomal enzymes that include proteases capable of digesting collagen.(8)
References:
2. Brown CC, Baker DC, Barker IK: Alimentary system. In: Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, pp. 20-21. Elsevier Saunders, Philadelphia, PA, 2007
3. Christensen H, Bisgaard M, Angen, O, Olsen JE: Final classification of Bisgaard taxon 9 as Actinobacillus arthritidis sp. nov. and recognition of a novel genomospecies for equine strains of Actinobacillus lignieresii. International Journal of Systematic and Evolutionary Microbiology 52:1239-1246, 2002
4. Henton MM, Van Der Lugt JJ: Actinobacillus Infections in Infectious Diseases of Livestock, eds. Coetzer JAW, Tustin, RC, 2nd ed., vol. 3, pp. 1648-1651 Oxford University Press, Cape Town South Africa, 2004
5. Lentsch RH, Wagner JE: Isolation of Actinobacillus lignieresii and Actinobacillus equuli from Laboratory Rodents. Journal of Clinical Microbiology 2:351-354, 1980
6. Rycroft AN, Garside LH: Actinobacillus species and their role in disease. The Veterinary Journal 159:18-36, 2000
7. Hussein MR: Mucocutaneous Splendore-Hoeppli phenomenon. Journal of Cutaneous Pathology 35:979-988, 2008
8. Abbas AK: Diseases of immunity. In: Pathological Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, 7th ed., pp. 213-214. Elsevier Saunders, Philadelphia, PA, 2005