Signalment:
After an uneventful 90-day quarantine period, one of the female fish from the recently-purchased group was bred with a male fish from the long-established colony. The resultant offspring were raised in several small groups up to the age of one month and then the groups were combined and maintained together in one large group in an 85-gal (~ 322 L) tank. At 13 months of age, eight fish from this group were selected at random for routine health assessment. These fish were euthanized by immersion in a solution of tricaine methanesulfonate (MS-222) and then the carcasses were fixed in toto in 10% neutral-buffered formalin. The fixed carcasses were then submitted for histopathologic examination. The submitted slide contains whole-body transverse sections from one of the eight fish. This fish was part of a research project conducted under an IACUC approved protocol in compliance with the Animal Welfare Act, PHS Policy, and other federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011.
Gross Description:
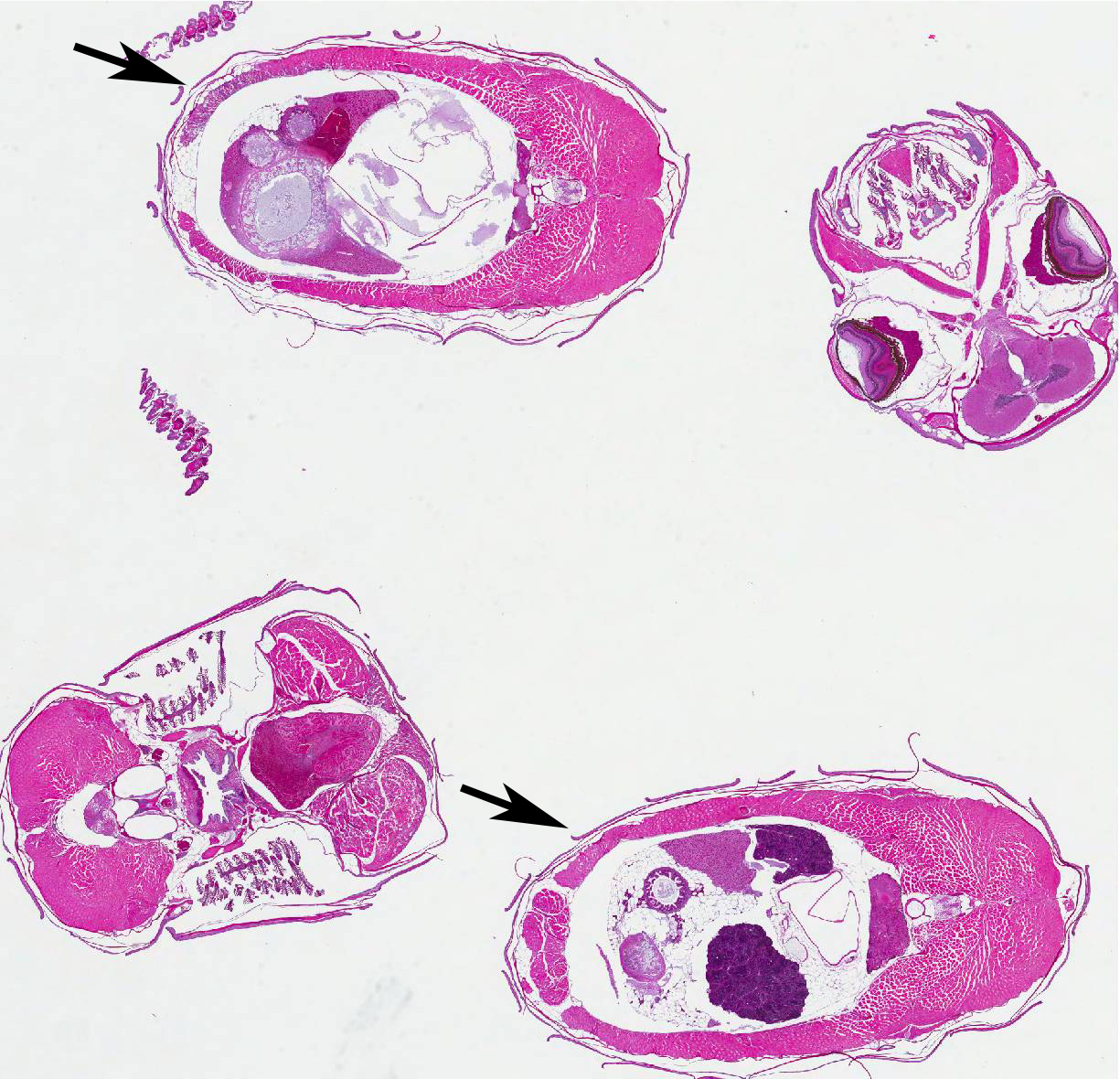
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
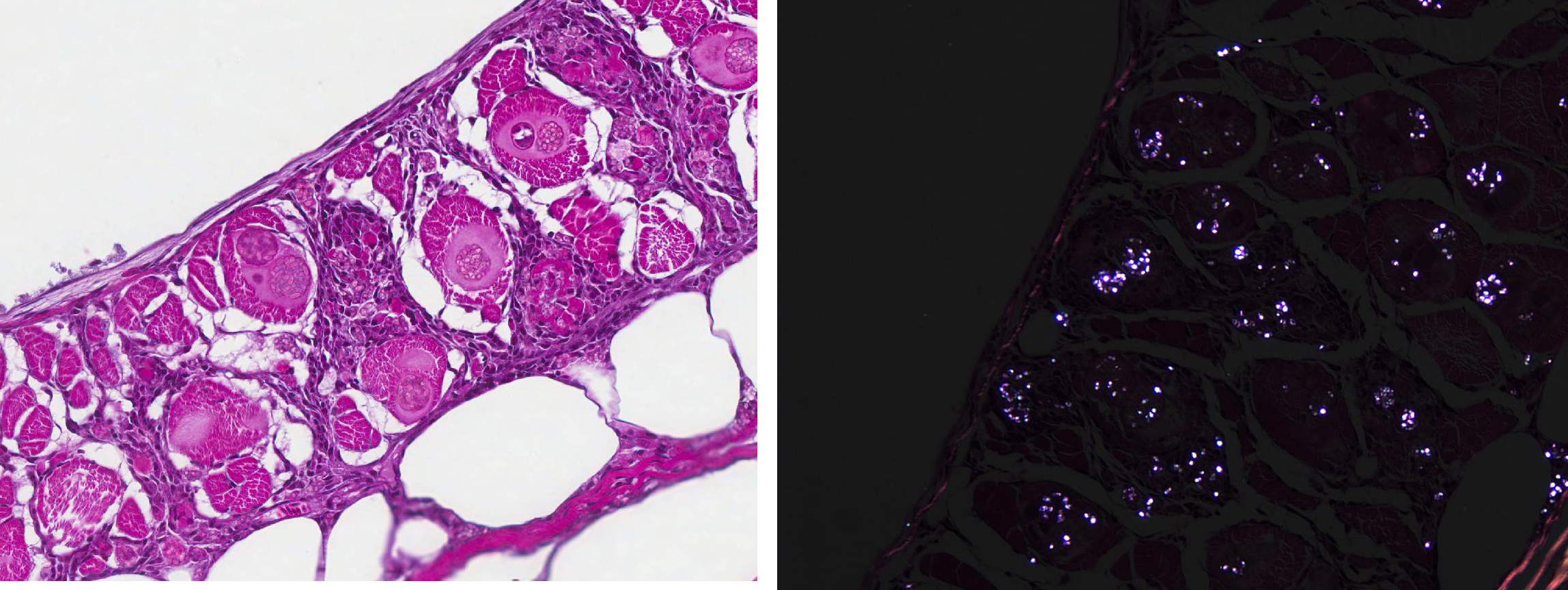
Several special stains were done in an attempt to better characterize the organisms. These stains revealed that the spores often had annular bands and a PAS-positive polar granule (Figure 6). The more mature spores were Gram-positive but immature spores were Gram-variable (Gram-negative or did not stain with Grams stain). These findings are characteristics of microsporidia.2,3,6
Further studies conducted by personnel at Oregon State University and the Zebrafish International Resource Center demonstrated that the species of microsporidia in this zebrafish is Pleistophora hyphessobryconis.7 P. hyphessobryconis is a common pathogen of neon tetras (Paracheirodon innesi ) and other species of ornamental tropical fish.5,6 P. hyphessobryconis is so common in neon tetras that the disease it causes is simply called neon tetra disease.5,6 Although some older reports state that P. hyphessobryconis has been seen in zebrafish in the commercial pet trade, this case was the first time it was recognized in a laboratory research colony.7 Subsequent to the discovery of this index case, other cases of P. hyphessobryconis infection in zebrafish have been seen in two other research colonies.7
For many years, microsporidia were taxonomically classified as protozoa. However, the results of more recent studies have caused them to be re-classified as fungi.2,4 Microsporidial spores are the infectious stage and each spore contains a coiled structure called a polar filament or polar tube.1 When a spore enters a suitable host (usually by ingestion of the spore), the polar filament uncoils, emerges from the spore, and penetrates a host cell. The sporoplasm is then injected through the polar filament into the host cell.2 The parasite proliferates asexually inside the host cell, first through merogony and then sporogony.3,6 The meronts of Pleistophora spp. (which at first are uninucleate and eventually become multinucleate) are surrounded by a membrane derived from the host cell and are said to reside within a parasitophorous vacuole.2,5 The meronts of Pleistophora spp. mature into sporogonial plasomodia that undergo fission to produce sporoblasts which ultimately develop into spores; multiple spores are contained within each thick-walled sporophorous vesicle.2,5
Fish typically become infected by P. hyphessobryconis by ingesting spores released into the environment from necrotic muscle fibers of other infected fish or by scavenging dead, infected fish. It is not known how the fish of this report was exposed to P. hyphessobryconis spores. This animal had been raised in a laboratory setting without any exposure to neon tetras or other fish species. The water used to house this fish colony is a mixture of deep-well water and municipal water that has undergone reverse-osmosis filtration; thus, it is highly unlikely that this water was the source of infection. The food used to feed the fish is a commercial flake-food that contains ground fish meal. However, the processing procedures used to produce this food should have inactivated any micro-sporidia spores that were accidentally present.
The mother of this fish had been purchased from a commercial vendor and thus may have been exposed to infected fish before her arrival at the research facility. Although the mother fish was clinically normal, it is possible that she was harboring a low-grade infection and thus may have shed spores into the water during breeding. However, the embryos produced from this breeding were treated with bleach before being transferred to fry-raising tanks, and this bleaching process should have inactivated any micro-sporidia spores present in the water. It is also worth noting that the affected fish was euthanized 13 months after conception and this would be a long time to harbor a subclinical infection.
When this infected fish was detected, all of the fish that had been purchased from the commercial vendor had been removed from the colony months previously. Thus, it was not possible to examine them to determine if they had been the source of infection. After this fish was found to be infected, the remaining 135 fish in its sibling co-hort were euthanized and examined grossly (98 fish) and/or histologically (37); none of these were found to be infected and there have not been any other cases in this colony since then. Note: Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.
JPC Diagnosis:
Conference Comment:
The most commonly encountered infectious diseases of laboratory zebrafish include mycobacteriosis and microsporidiosis.7,8 As mentioned by the contributor, based on phylogenetic analysis and the presence of chitin within the spore, microsporidia are classified as fungal-like organisms rather than protozoans, as previously thought. Microsporidia are a large group of obligate-intracellular eukaryotic parasites that infect a wide range of animal species.1,3 Their relatively simple life cycle consists of two developmental stages: merogony and sporogony.1,2,3,5,7,8 After infection, meronts multiply within the host cell, skeletal muscle in this case, eventually forming thick-shelled spores. Spores are released from the ruptured host cell and reach the environment through various bodily secretions. The infectious spores have a thick, chitinous shell and are resistant to environmental stress; thus allowing the infective spores to remain viable in the aquatic environment for a prolonged period of time. As mentioned by the contributor, infective spores can also be ingested through predation.7,8
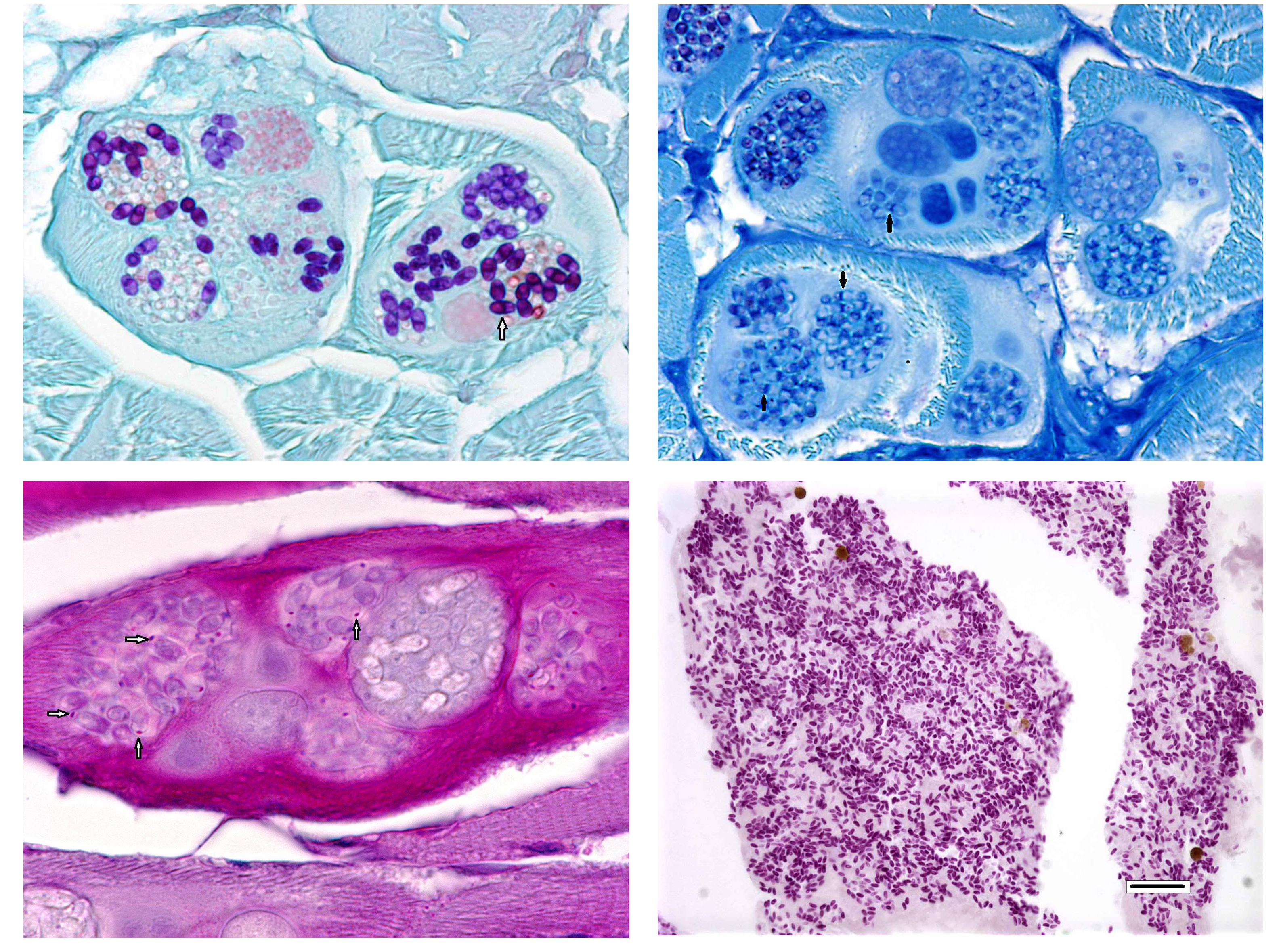
The most commonly identified micro-sporidian parasite affecting zebrafish is Pseudoloma neurophila.7,8 As is implied by the name, P. neurophila have tropism for the central and peripheral nervous system and is associated with encephalomyelitis and polyneuritis. Small groups and individual spores can be seen in a variety of extraneural organs, including smooth and skeletal muscle. Spores released from peripheral nerves and skeletal muscle typically cause severe inflammation.7,8 In contrast, Pleistophora hyphessobryconis primarily develops within skeletal muscle with multiple developmental stages (meronts, sporoblasts, and spores) present within the sarcoplasm. The conference moderator noted that the two Microsporidia species can be easily distinguished histologically due to the presence of P.hyphessobryconis intra-sarcoplasmic developmental stages and thick-walled sporophorous vacuoles laden with spores.7,8 Additionally, spores of P. hyphessobryconis are associated with only a mild inflammatory response, as seen in this case. In the areas of mild histiocytic inflammation in the skeletal muscle of the abdominal wall, birefringent spores are present within phagocytes. In photographs provided by the contributor, wet-mount preparations of skeletal muscle from infected fish show individual spores as well as sporophorous vacuoles containing spores with a prominent posterior vacuole and coiled polar filament, typical of Microsporidia spp.1,7,8 Another micro-sporidium parasite of veterinary importance is Encephalitozoon cuniculi, which causes torticollis and phaecoclastic uveitis in the highly susceptible dwarf rabbit.1 Readers are encouraged to review for an outstanding example of phaecoclastic uveitis caused by E. cuniculi in a double-maned lionhead rabbit.
References:
1. Cantile C, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 1. St. Louis, MO: Elsevier; 2016:385.
2. Curry A. Microsporidiosis. In: Cox FEG, Wakelin D, Gillespie SH, Despommler DD, ed. Topley & Wilsons Microbiology & Microbial Infections Parasitology. 10th ed. London, UK: Hodder Arnold, 2005: 529-535.
3. Gardiner CH, Fayer R, Dubey JP. An Atlas of Protozoan Parasites in Animal Tissues. 2nd ed. Washington, DC: Armed Forces Institute of Pathology, 1998.
4. Lee SC, Corradi N, Byrnes EJ 3rd, et al. Microsporidia evolved from ancestral sexual fungi. Curr Biol. 2008;18(21):1675-9.
5. Li K, Chang O, Wang F, Liu C, Liang H, Wu S. Ultrastructure, development, and molecular phylogeny of Pleistophora hyphessobryconis, a broad host microsporidian parasite of Puntius tetrazona. Parasitol Res. 2012;111:17151724.
6. Post G. Textbook of Fish Health. 2nd ed. Neptune City, NJ: T.F.H Publications, Inc., 1987.
7. Sanders JL, Lawrence C, Nichols DK, et al. Pleistophora hyphessobryconis (Microsporidia) infecting zebrafish Danio rerio in research facilities. Dis Aquat Org. 2010;91:47-56.
8. Sanders JL, Watral V, Kent ML. Microsporidiosis in zebrafish research facilities. ILAR J. 2012; 53(2):106-113.