Signalment:
17-year-old gelded Westfalian warmblood, horse (
Equus caballus).The horse had an eight-week history of pneumonia. Physical examination revealed a reduced nutritional status, and a purulent exudate was detected within the trachea. Microbiological investigation yielded growth of
Aspergillus fumigates within the tracheal fluid. Based on the thoracic radiography results, a granulomatous pneumonia was suspected. Due to unsuccessful treatment, the horse was eventually euthanized for animal welfare reasons.
Gross Description:
The horse was in a reduced nutritional condition. Necropsy revealed that the main lesions were restricted to the lung. Lung parenchyma was interspersed with multiple large, bulging, coalescent, tan to white and firm nodules of fibrosis affecting all lobes. The patchy areas of fibrosis had predominantly distinct borders to the adjacent normal appearing lung tissue. The cut surfaces of the nodules were homogeneously tan to white. Numerous subpleural hyperemic blood vessels and emphysema of the cranial lung lobes were additionally detected. The bronchial lymph nodes were noticeably enlarged. Furthermore, the trachea revealed a streaky redness of mucosa.
Histopathologic Description:
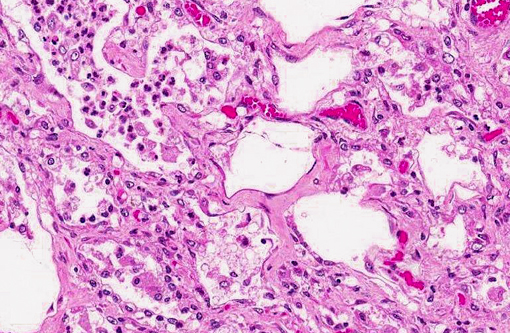
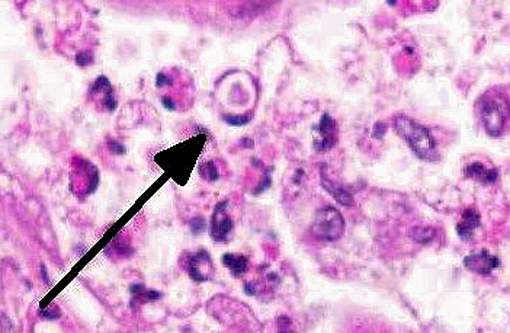
The lung fibrosis resulted in a vast interstitial expansion and eventually led to a complete remodeling of the lung architecture and formation of abnormal cystic airspaces (honeycombing). These airspaces were lined by a cuboidal epithelium. According to the gross findings, the nodular lesions were sharply demarcated from normal parenchyma. The interstitium was infiltrated by numerous inflammatory cells including lymphocytes, plasma cells and neutrophils as well as some eosinophils and multinucleated giant cells. The bronchioli, alveoli, and abnormal cystic airspaces were filled with numerous large vacuolated macrophages and neutrophils. Occasionally, some multinucleated giant cells were detected. Within lung areas with chronic inflammatory lesions, a few enlarged intraluminal macrophages were found that showed eosinophilic intranuclear inclusion bodies (Cowdry type A). Some lung areas revealed a marked alveolar edema and hyperplasia of type II pneumocytes. Special stains for bacteria and fungi yielded negative results. The bronchiolar lymph nodes were severely hyperplastic. Acute hemorrhages were found within submucosal and muscular layers of the trachea. No inflammation and no pathogenic agents were detectable within the tracheal mucosa using special staining methods.
Morphologic Diagnosis:
Lung: Equine multinodular pulmonary fibrosis (EMPF).
Lab Results:
Lung: DNA of both equine herpesvirus type 5 (EHV-5) and EHV-2 was detected using standard PCR analysis.
Tracheal fluid: mild growth of
Candida species.
Condition:
Equine Multinodular Pulmonary Fibrosis (EMPF)
Contributor Comment:
Equine multinodular pulmonary fibrosis (EMPF) is a chronic and fibrosing interstitial lung disease in adult horses that results in characteristic lung lesions. The disease was first recognized in 2005 by Williams et al.(1) and further cases in the USA and Europe have recently been described.(2-5) In all reported cases, the disease was associated with an infection of EHV-5, which belongs to the genus percavirus that is actually classified within the gammaherpesvirus subfamily.(6) A co-infection with EHV-2 was detected in several cases, but it seems not to be associated with EMPF.(2,3,5) In horses suffering from EMPF, the lung lesions differ significantly from other interstitial fibrosis in lung diseases due to their nodular pattern, the parenchymal remodeling with honeycombing and the presence of intranuclear viral inclusion bodies within intraluminal macrophages. Other noxa, such as thermal and chemical injury, toxic gases, ingested toxins, endotoxins, pneumoconiosis, uremia and chronic left heart failure could be excluded in the present case as cause for interstitial pneumonia. The pathogenesis of EMPF is unclear at the moment.
JPC Diagnosis:
Lung: Fibrosis, interstitial, diffuse, severe, with histiocytic and neutrophilic alveolitis, type II pneumocyte hyperplasia and rare eosinophilic intrahistiocytic intranuclear viral inclusions.
Conference Comment:
As noted by the contributor, Kochs postulates have not yet been fulfilled to definitively establish EHV-5 as the etiology of EMPF. Moreover, asinine herpesvirus-5 (AHV-5) has also been detected by PCR either alone, or in combination with EHV-5, in some cases of EMPF, suggesting that other herpesviruses may be involved in the pathogenesis of the disease. An analogous human condition, idiopathic pulmonary fibrosis (IPF) is similarly attributed to an array of potential causes, including pneumocyte injury, abnormal fibroplasia, inflammation, and the excessive deposition of extracellular matrix proteins. As with EMPF, gammaherpesviruses have been implicated in the pathogenesis and/or exacerbation of IPF, as well as IPF-like lesions in mice.(7)
Conference participants discussed the importance of the cytokine milieu in driving macrophage activation and expression, and speculated that, based on the degree of fibroplasia that typifies EMPF, alternative (M2) activation of macrophages is most likely to predominate in this condition. Monocytes exposed to the signature cytokines of Th2 lymphocytes (i.e., IL-4 and IL-13) undergo alternative (M2) activation to mature into a fibrotic phenotype and produce polyamines that drive cell proliferation, and proline and TGF-β that drive collagen production.(8)
Other notable gammaherpesviruses of importance in animals were briefly reviewed, including those associated with IPF-like lesions in the lungs of mice (i.e., Murine herpesvirus-68), urogenital carcinomas in California sea lions (i.e., Otarine herpesvirus-1), and malignant catarrhal fever in ruminants (i.e., Alcelaphine herpesvirus-1, Alcelaphine herpesvirus-2, Hippotragine herpesvirus-1, Ovine herpesvirus-2, Caprine herpesvirus-2 and MCFV-white tailed deer).
References:
1. Williams KJ, Jackson CA, Scott MA, Robinson NE, Derksen FJ, Macs R, et al. Multinodular equine pulmonary fibrosis: a newly recognized herposvinis associated respiratory disease of horses. ACVP/ASVCP meeting.
Vet Pattie'. 2005;42:716.
2. Williams KJ, Maes R, Del Piero F, Lim A, Wise A, Bolin DC, et al. Equine multinodular pulmonary fibrosis: a newly recognized herpesvirus-associated tihtotic lung disease.
Vet Pathol. 2007;44:849-862.
3. Hart KA, Barton MH, Williams KJ, Flaminio MJBF, Howerth EW. Pulmonary fibrosis, pancytopenia and equine herpesvirus-5 infection in a Thoroughbred gelding.
Equine Vet Educ. 2008;20:470-6.
4. Wong DM, Belgrave RL, Williams KJ, Del Piero F, Alcott CJ, Bolin SR, et al. Multinodular pulmonary fibrosis in five horses.
J Am Vet Med Assoc. 2008;232:898-905.
5. Poth T, Niedermaier G, Hermanns W. Equine multinodular pulmonary fibrosis (EMPF) in association with an EHV-5 infection in 5 horses.
Wien Tierailt1 Monatsschr. 2009;96:203-8.
6. McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution.
Virus Res. 2006;117:90-104.
7. Back H, Kendall A, Grandon R, Ullman K, Treiberg-Berndsson L, Stahl K, et al. Equine multinodular pulmonary fibrosis in association with Asinine herpesvirus type 5 and Equine herpesvirus type 5: a case report.
Acta Veterinaria Scandinavica. 2012;54:57.
8. Ackermann MR. Inflammation and healing. In: Zachary JF, McGavin MD, eds.
Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012: 128-130.