Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 6
2 October 2019
Dr.
Jeff Wolf, DVM
Senior Pathologist
Chief Scientific Officer/Pathology Manager
EPL, Inc.
Sterling, VA
CASE II: AP 17-4634 (JPC 4120175).
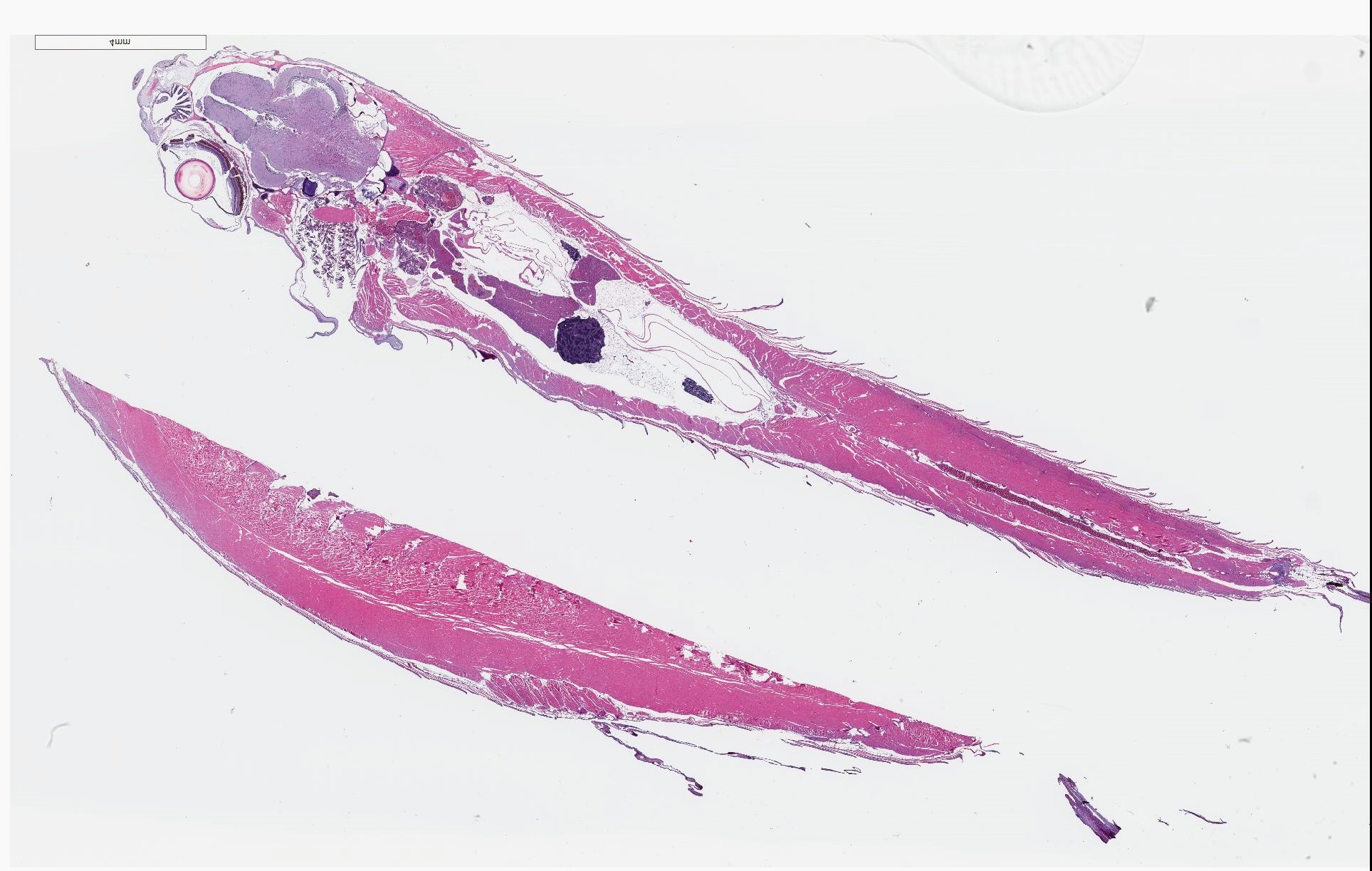
Signalment: 10-month-old male wildtype line (unspecified), zebrafish, Danio rerio
History: A wild-type control zebrafish for a leukemia study was swimming in circles and removed from the study for pathology evaluation.
Gross Pathology: No notable gross findings were observed.
Laboratory results: N/A
Microscopic Description:
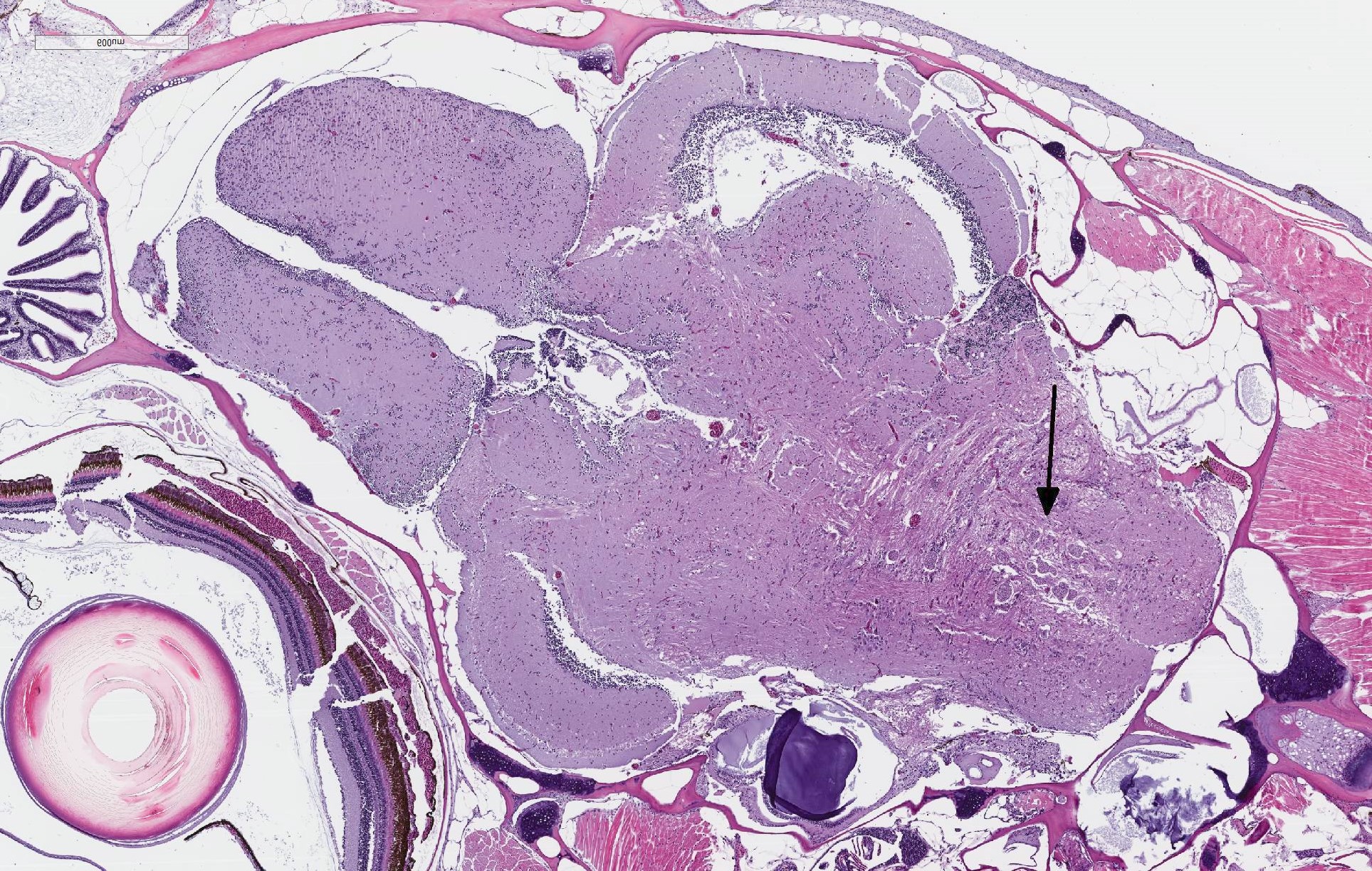
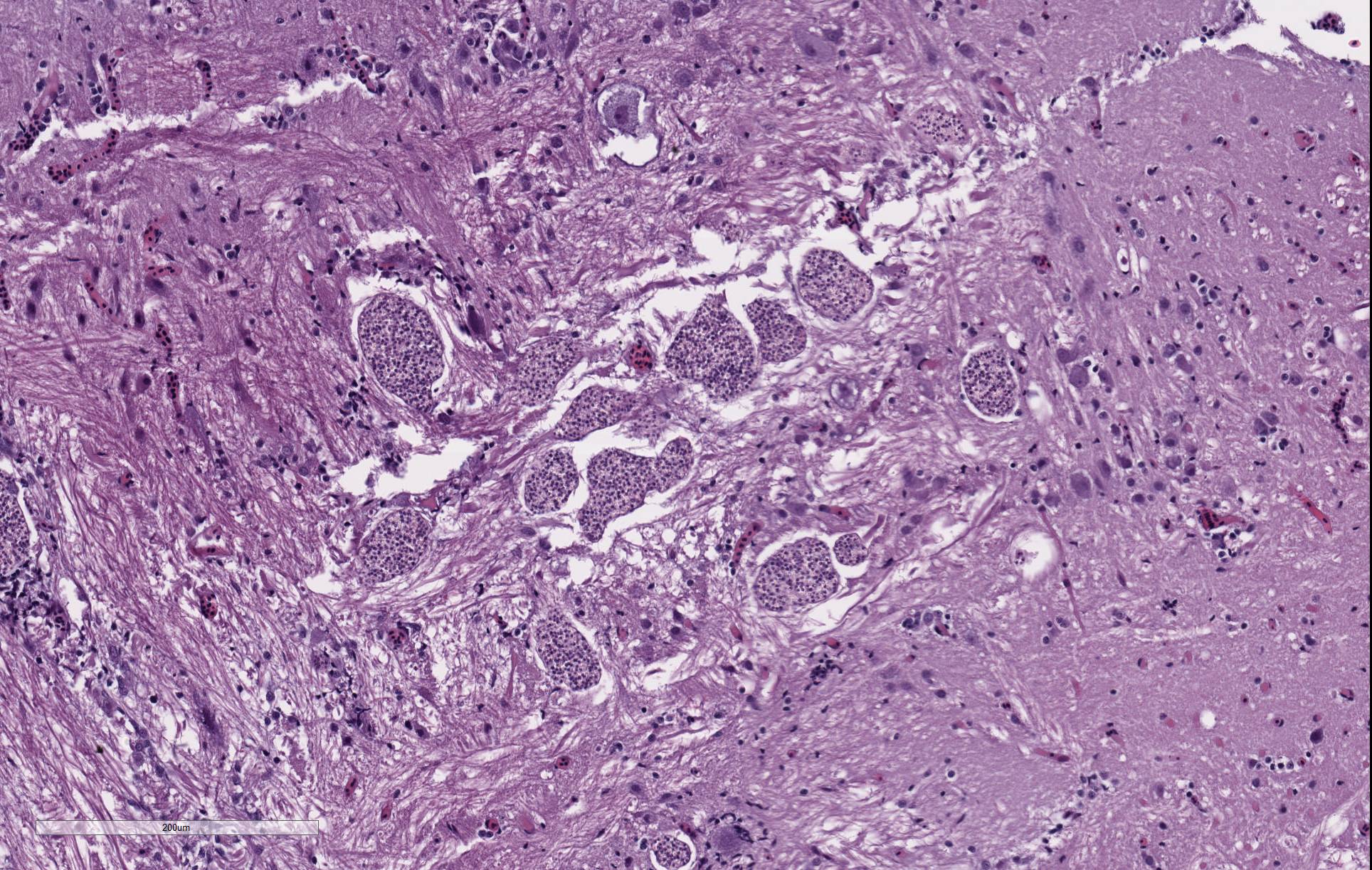
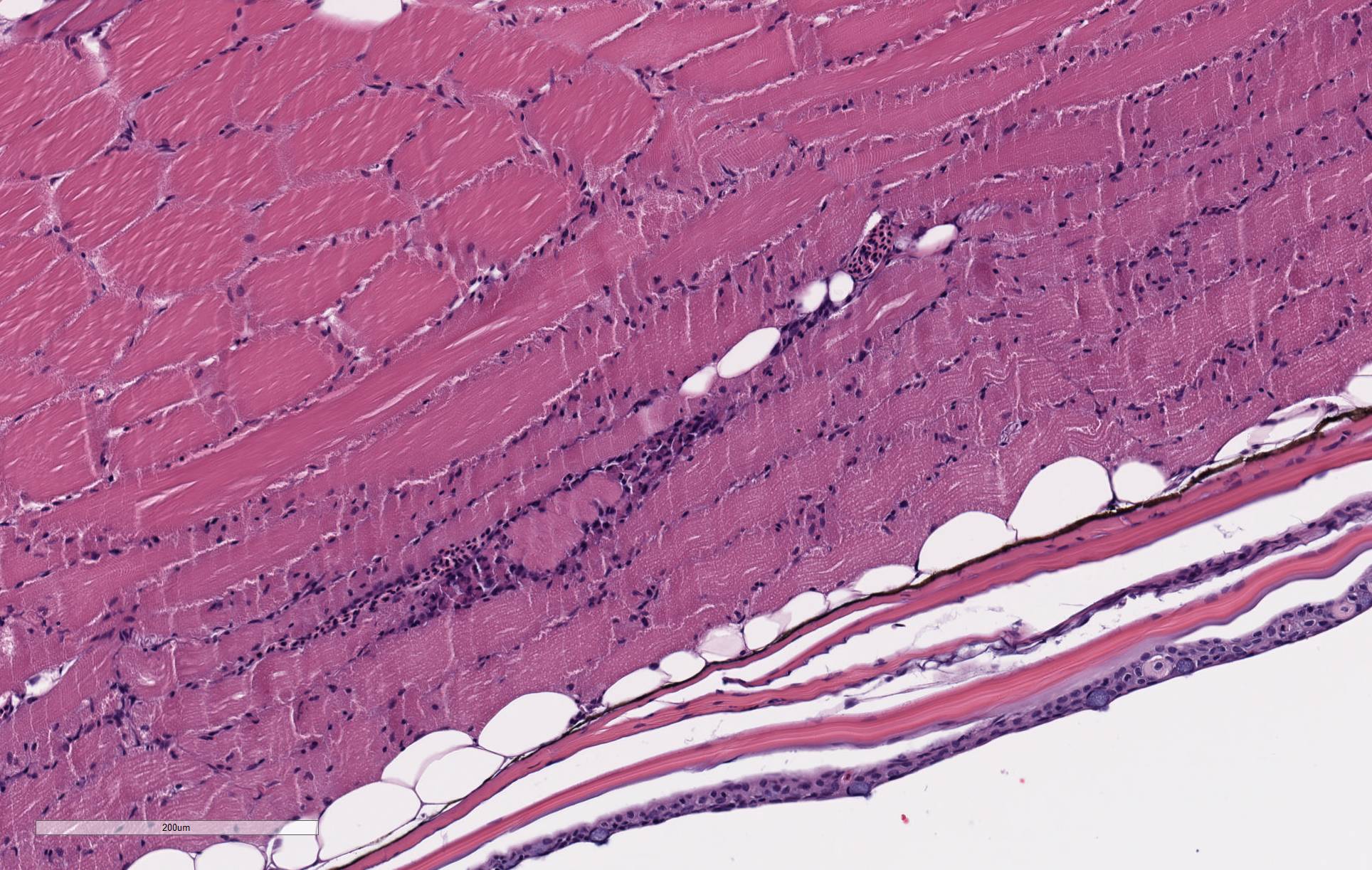
There are randomly distributed and variably sized cross-sections of parasitic complexes containing numerous spores within the white mater of the posterior brain and brain stem. The majority of parasitic spores appear as uninucleate structures by H&E staining and are approximately 3.0 µM wide x 5.0 µM long with posterior vacuoles. Low numbers of glial cells are found at the periphery of some complexes while the neuropil appears devoid of cells at other locations were complexes are observed. Spores were not detected in other sampled tissues.
Contributor Morphologic Diagnosis:
Brain, neuropil: Parasitic complexes, multifocal, with morphology that is most consistent with Pseudoloma neurophilia infection.
Contributor Comment: Pseudoloma neurophilia is a commonly encountered and well described microsporidian parasite of zebrafish. Unlike other microsporidia, P. neurophilia does not appear to form xenomas. Histologic and ultrastructural studies of oral exposure to the proliferative parasitic stages show that early developmental phases interface with zebrafish host cells through a glycocalyx-like coating. Sporophorous vacuoles are then formed where the sporogonic stages remain to undergo karyokinesis, producing tetranucleate stages that then divide into uninucleate sporoblasts and spores. Spores remaining within these parasite complexes may or may not elicit an immune response.1
Recent studies are better clarifying the genomic basis of P. neurophila infections as well as the effects of silent infections on research.1,4,8-10 P. neurophilia may be transmitted either through a vertical or horizontal route. It often presents in zebrafish colonies as a chronic, subclinical infection that primarily affects the brain and skeletal muscle.5 Infected fish demonstrate a range of presentations included altered behavior, reduced growth or spinal deformities (e.g., lordosis and kyphosis). Alternatively, there may be no visible indications of infections and only baseline mortality rates for a facility. The variable clinical presentations and the potential for false negatives using either histology or molecular testing when conducting sentinel surveillance makes eradication of microsporidial infections challenging. Additionally data gathered from fish from infected colonies has the potential for inconsistent results for behavioral, immunology and hematopoiesis studies. The negative effects of microsporidial infections in colonies used for research studies is especially profound when immunosuppressive therapies, like gamma irradiation, are used as parasitic infections often worsen. Interestingly aspects of the subclinical disease as well as understanding how these infections become clinically significant following immunosuppression in zebrafish are being advanced as one way to study aspects of microsporidial infections in humans.4,6,8,9
Contributing Institution:
Department of Pathology, College of Veterinary Medicine, University of Georgia, Athens, GA 30602, www.vet.uga.edu/VPP
JPC Diagnosis: Hindbrain:
Microsporidial xenomas, multiple.
2. Skeletal muscle: Degeneration and necrosis, multifocal.
JPC Comment: Pseudoloma neurophilium is a common microsporidial parasite of zebrafish. Transmission occurs vertically and horizontal routes of transmission are the result of consumption of environmentally resistant spores in water contaminated by carcasses and eggs. Standard sterilization techniques, such as bleaching, do not impact spores, and spores contained within embryonated eggs are shielded from disinfectants.9
A recent study also demonstrated reduced survivability of P. neurophila spores following cryopreservation.3 This is an important finding as cryopreservation of sperm is widely used in the maintenance and distribution of many strain type zebrafish used in research. Other zebrafish pathogens examined in this fashion were Mycobacterium marinum and chelonae (minimal impact upon freezing and thawing), and eggs of Pseudocapillary tomentosa (no survival).3
Due to their social nature, zebrafish are used in a wide variety of behavioral research studies on human diseases, such as autism, schizophrenia, depression, and PTSD, as well as to study the behavioral effects of a wide variety of pharmaceuticals.8,11 Startle behavior, shoaling, and interfish distance of laboratory zebrafish are all interpretable responses in judging behavioral abnormalities. Fish infected with P. neurophilia have been shown to have abnorming shoaling behavior and interfish distance, which may potentially complicate behavioral studies using this animal model.11
A recent publication
from the research group at Oregon State University at Corvallis, one of the
leading groups in the area of zebrafish research, recently identified a number
of species of aquarium fish that may also be infected with P. neurophilia
in a mixed-species aquarium setting.7 While long considered to be
an infection restricted to Danio rerio, there are now five families and
eight species which have been identified to be infectable with this parasite. The
species include giant danio, medaka, fathead minnows, goldfish, platys, Siamese
fighting fish, and neon tetras.7
Contributing Institution:
St. Jude Children’s Research Hospital, Department of Pathology
https://www.stjude.org/research/departments-divisions/pathology.html
References:
1. Cali, A., et al., Development, ultrastructural pathology, and taxonomic revision of the microsporidial genus, Pseudoloma and its type species Pseudoloma neurophilia, in skeletal muscle and nervous tissue of experimentally infected zebrafish (Danio rerio). J Eukaryo Micro 2012; 59(1): 40-48.
2. Matthiew, JL, et al. Pseudoloma neurophilia n. g., n. sp., a new microsporidium from the central nervous system of the zebrafish (Danio rerio). J Eukaryo Micro 2001; 48(2):227-233.
3. Norris, LJ, Watral V, Kent ML. Survival of bacterial and parasitic pathogens from zebrafish (Danio rerio) after cryopreservation and thawing. Zebrafish 2018; 15(2):188-201.
4. Ramsay JM, et al., Pseudoloma neurophilia (Microsporidia) infections in zebrafish (Danio rerio): effects of stress on survival, growth and reproduction. Dis Aquatic Org 2009. 88(1):69-84.
5. Sanders JL et al., Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the zebrafish, Danio rerio. PLoS ONE, 2013; 8(9):e76064.
6. Sanders, J.L., V. Watral, and M.L. Kent. Microsporidiosis in zebrafish research facilities. ILAR Journal / National Research Council, Institute of Laboratory Animal Resources, 2012; 53(2): p. 106-113.
7. Sanders JL, Watral V, Stidworthy MF, Kent ML. Expansion of the known host range of the microsporidium, Pseudoloma neurophilia. Zebrafish 2016; Supp 1:S102-S107.
8. Spagnoli, S., L. Xue, and M.L. Kent, The common neural parasite Pseudoloma neurophilia is associated with altered startle response habituation in adult zebrafish (Danio rerio): Implications for the zebrafish as a model organism. Behavioural brain research, 2015. 291: p. 351-360.
9. Spagnoli, S.T., et al., Pseudoloma neurophilia infection combined with gamma irradiation causes increased mortality in adult zebrafish (Danio rerio) compared to infection or irradiation alone: new implications for studies involving immunosuppression. Zebrafish, 2016. 13(Suppl 1): p. S-107-S-114.
10. Spagnoli, S.T., et al., Pseudoloma neurophilia: A retrospective and descriptive study of nervous system and muscle Infections, with new implications for pathogenesis and behavioral phenotypes. Zebrafish 2015; 12(2):189-201.
11. Spagnoli S, Sanders J, Kent M. The common neural parasite Pseudoloma neurophilia causes altered shoaling behavior in adult laboratry zebrafish and its impoicatiobs for neurobehavioral research. J Fish Dis 2017; 40(3):443-446.