Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 5
Sept 19th, 2018
CASE IV: Case 2 (JPC 4068765-00).
Signalment: 6 month old, female, Harlan Sprague-Dawley rat, (Rattus norvegicus)
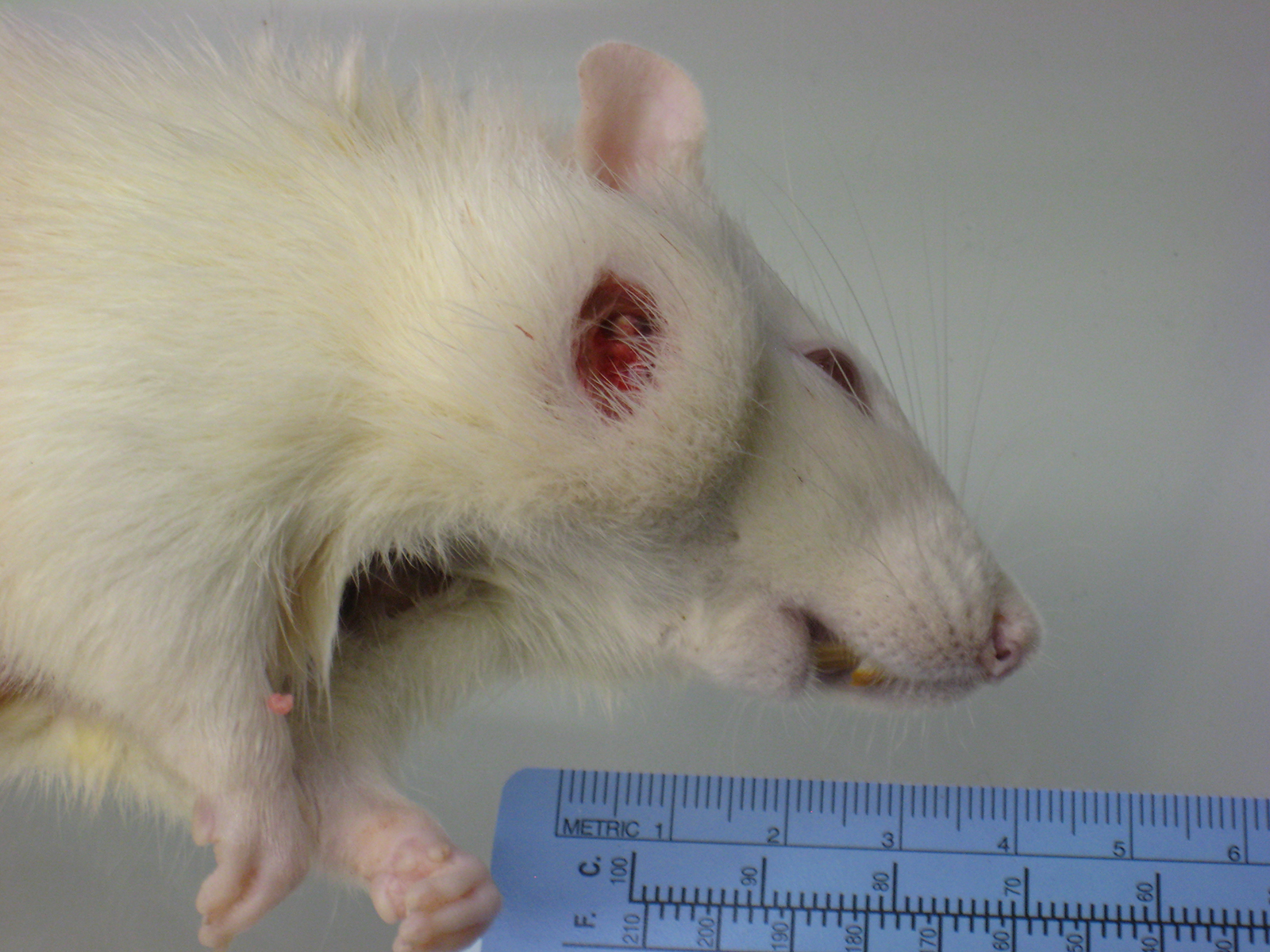
History: Moribund sacrifice due to ulcerated facial mass; several animals affected.
Gross Pathology: A multinodular tan mass replaced and destroyed most of the left submandibular salivary gland. After section the mass, measuring 2cmx1cm, appeared encapsulated and filled with white to tan caseous material.
Laboratory results: None provided.
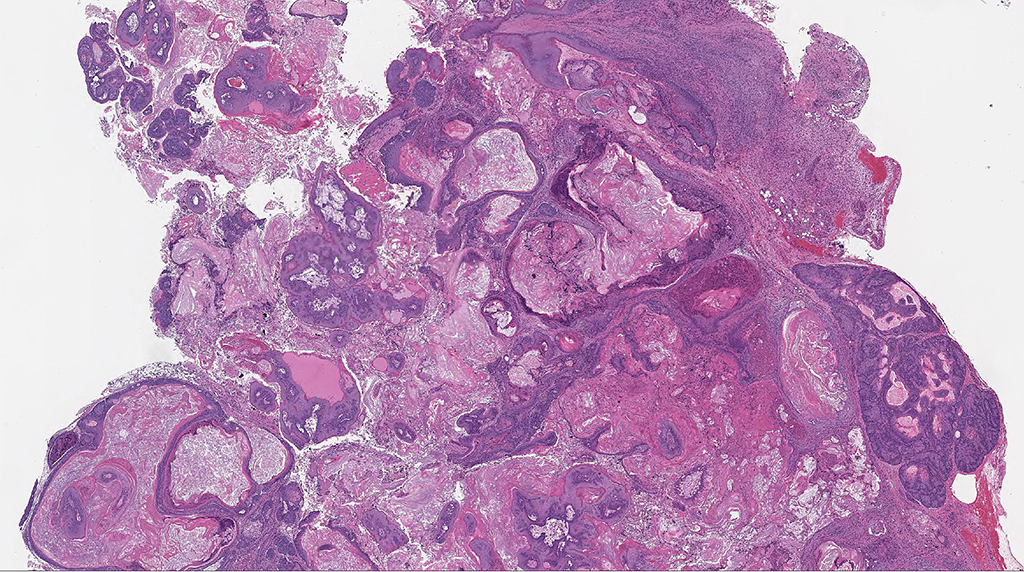
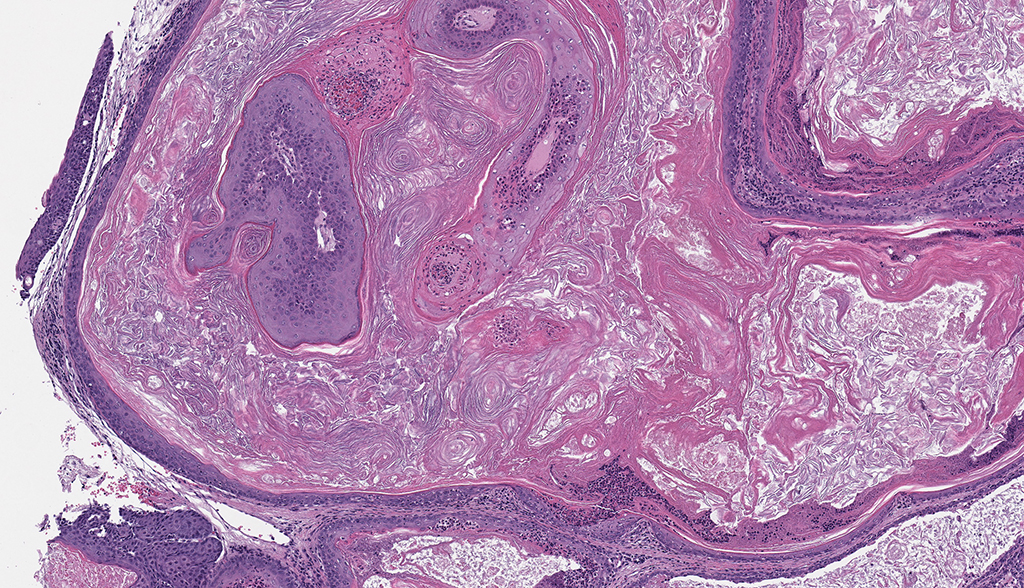
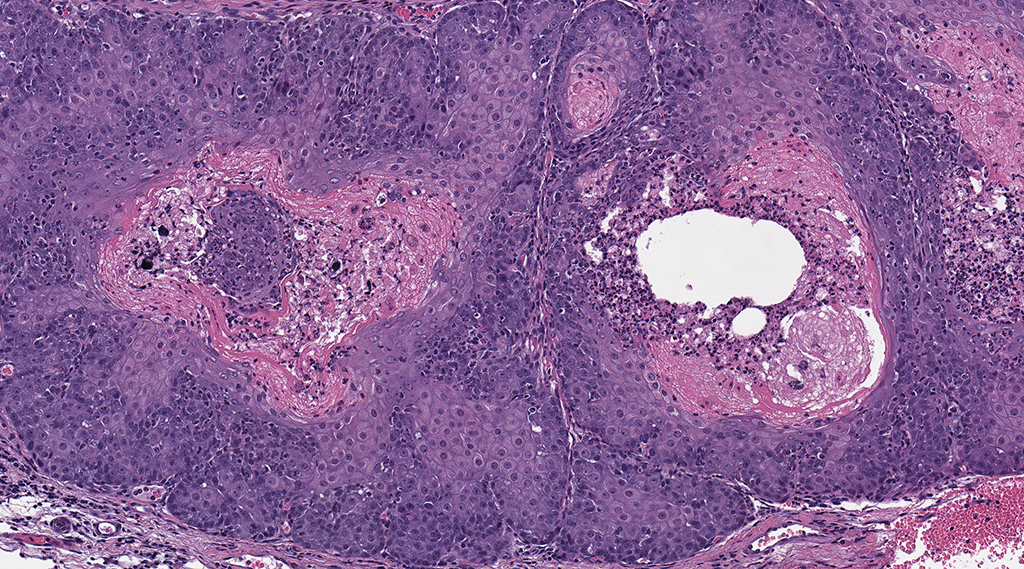
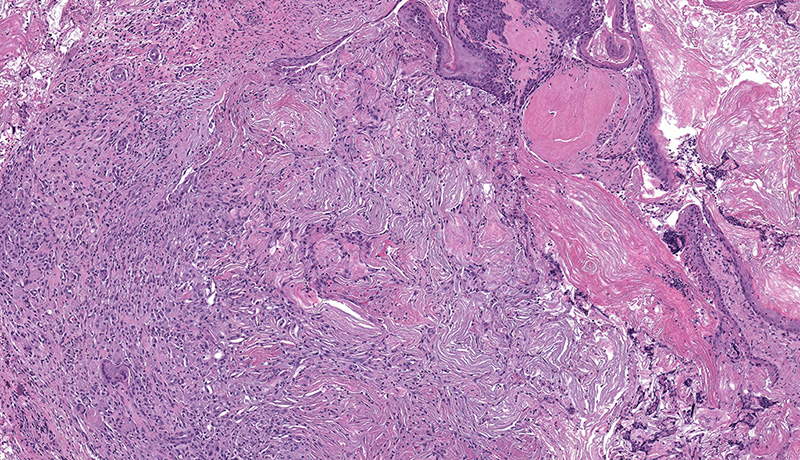
Microscopic Description: Facial mass: An approximately 2 x 2cm, well-circumscribed, unencapsulated, multilobular, and cystic neoplasm is expanding and compressing the subcutis. The neoplasm is composed of polygonal cells arranged in islands, cords, and trabeculae supported by moderate fibrovascular stroma. Neoplastic islands and trabeculae are often bordered by a peripheral layer of flattened to cuboidal epithelial cells with scant, eosinophilic cytoplasm, and condensed round to oval nuclei (basaloid reserve cells) and also contain a central area comprised of polygonal cells with abundant, microvacuolated cytoplasm and round nuclei with finely stippled chromatin and 1-3 variably distinct nucleoli (sebaceous differentiation). Anisocytosis and anisokaryosis are minimal and the mitotic rate for both cell populations is less than 1 mitotic figure per 40X field. Also within the neoplasm, are multifocal areas of squamous epithelial cells undergoing gradual keratinization, lining cystic spaces filled with lamellar whorls of keratin, moderate numbers of viable and degenerative neutrophils, smooth proteinaceous material (sebum), and variable amounts of necrotic debris (cystic degeneration and ductal differentiation). The neoplasm is focally disrupted and replaced by large numbers of epithelioid macrophages with scattered neutrophils and multinucleated giant cells (pyogranulomatous inflammation), reactive fibroblasts with perpendicularly arranged small caliber vessels lined by hypertrophied endothelium (granulation tissue) admixed with collagen, lamellations of keratin, and necrotic and proteinaceous debris. Some of these areas appear to be associated with focally extensive ulceration. There are also multifocal areas of mild hemorrhage scattered throughout the neoplasm.
Contributor’s Morphologic Diagnoses: Zymbal’s gland: Adenoma.
Contributor’s Comment: The Zymbal’s gland or auditory sebaceous gland is a specialized sebaceous gland unique to rodents and insectivores.3,6,7 This compound branched acinar gland sits anterioventral to the external ear meatus and is composed of 3-4 triangular lobes. These lobes are characterized by clusters of saccular acini surrounding a prominent interlobular duct that drains into a short excretory duct. The periphery of each acinus is lined by an incomplete layer of flattened to cuboidal cells containing hyperchromatic nuclei and scant cytoplasm (basaloid reserve cells) while the center is comprised of large polygonal cells with pale, foamy cytoplasm due to the progressive accumulation of lipid vacuoles.6 Two smaller sebaceous glands underlying the ear canal epithelium are sometimes considered to be a component of the Zymbal’s gland; these glands are holocrine glands, as their secretory products are formed from degenerative mature acinar cells that disintegrate and discharge into the ducts.3,4,6
Spontaneous neoplasms of the Zymbal’s gland are uncommon in Fisher 344 (F344) and Harlan-Sprague Dawley rats and rarely occur in the mouse.1,5,6 The classic presentation for a Zymbal’s gland tumor is an ulcerated mass on the side of the face or the base of the ear. Early neoplasms are typically firm, well-circumscribed, freely moveable, subcutaneous masses whereas larger neoplasms are frequently ulcerated, with or without extension into the ear canal, and may exude caseous or purulent material on cut section. Most Zymbal’s gland neoplasms exhibit histological features of malignancy with local invasion, cellular atypia, necrosis and high mitotic activity, however, metastasis is not reported to occur. 3,4,6 Benign neoplasms, adenomas or papillomas, are generally smaller, well-circumscribed masses with minimal cellular atypia and invasion. Zymbal’s gland carcinomas are less well-circumscribed with invasion of the surrounding tissues and frequent cystic cavities filled with mixtures of proteinaceous fluid (sebum), keratin and necrotic debris. The neoplastic sebaceous and squamous cells that comprise the malignant neoplasms are very pleomorphic and anaplastic and some tumors are composed almost entirely of squamous epithelium.4,6
While spontaneous Zymbal’s gland neoplasms are infrequently observed, these types of tumors are easily induced by a variety of carcinogens, particularly aromatic amines like benzene.3,4,7 The Zymbal’s gland lacks transacylase and sulfotransferase activity, but is able to hydroxylate compounds via cytochrome p450-dependent enzymatic pathways. Detectable cytochrome p450 activity within these tumors suggests that the formation of active metabolites within the affected gland may contribute to tumorigenesis. In a survey conducted by the National Cancer Institute, 8% of rats treated with carcinogenic compounds developed Zymbal’s gland neoplasms (where N = 526 different chemical studies), yet tumor incidence increased to 14% among rats treated with those chemicals known to be mutagenic for Salmonella typhimurium (N = 214); this indicates that those chemicals typically causing neoplastic transformation in specialized sebaceous glands, like the Zymbal’s gland, are typically mutagenic compounds.2,6 The rat in this case was part of an 18-month carcinogenicity study for a known carcinogen with no reported mutagenic activity.
JPC Diagnosis: Zymbal’s gland: Adenoma.
Conference Comment: Due to their unique location and fairly characteristic histologic presentation, Zymbal’s gland neoplasms are one of the most well-known neoplasms in the rat. The contributor has provided a concise but thorough review of Zymbal’s gland neoplasia in the rat. However, it should be noted that the Zymbal’s gland undergoes other degenerative, inflammatory, vascular and other non-neoplastic changes, most similar to those observed in the mammary gland.
Non-neoplastic changes of degeneration, single cell necrosis (apoptosis), necrosis, and regeneration changes may all be seen in the ductal and sebaceous epithelium4 following administration of toxins, and cystic degeneration of the ducts may be seen as an aging change. Zymbal’s glands are uncommonly inflamed, but may be home to the typical range of inflammatory cells, largely depending upon the chronicity of the insult. It may be a site for metastasis in lymphoma or other neoplasms of hematopoietic origin.4 Both hyperplastic and atrophic changes are occasionally observed in acinar epithelium. The retention of normal tissue architecture aids in differentiating adenoma from hyperplastic lesions, and adenomas will also have increased numbers of basal cells.4
Neoplasms of Zymbals’s glands include adenomas (as seen in this case), carcinomas, and squamous papillomas, which may closely resemble tumors of the glandular epithelium, and often pose a diagnostic dilemma.
Carcinomas may be differentiated from adenomas due to the presence of large, irregular acini lacking ducts, and the presence of invasive growth with nests or cords of squamous epithelium penetrating the basal lamina and invading the underlying dermis and skeletal muscle.4 Close examination will reveal abnormalities in cellular differentiation of cells above the basal layer; there is variation in size and staining of nuclei, atypical mitotic figures, and loss of intracellular bridges.4
A more difficult differential diagnosis is squamous papilloma of the Zymbal’s gland (WSC 2015-2016, Conference 22, Case 3.) These neoplasms arise from the main duct epithelium and have a complex arborizing structure with maturing squamous epithelium overlying fibrous cores. One of the keys for the diagnosis of squamous papilloma of the Zymbal’s gland is the absence of sebaceous differentiation and the entirely keratinaceous nature of the contents in the cystic area of the neoplasm. 4
Contributing Institution:
NIEHS/NTP
References:
- Brix A, Nyska A, Haseman JK, Sells DM, Jokinen MP, Walker NJ. Incidences of Selected Lesions in Control Female Harlan Sprague–Dawley Rats from Two-Year Studies Performed by the National Toxicology Program. Toxicologic Pathology 2005:33:477-483.
- Gold LW, Manley NB, Slone TH, Ward JM. Review Article: Compendium of Chemical Carcinogens by Target Organ: Results of Chronic Bioassays in Rats, Mice, Hamsters, Dogs, and Monkeys. Toxicologic Pathology 2001:29:639-352.
- Percy DH and Barthold SW. Rat: Zymbal’s gland tumors. In: Percy DH and Barthold SW, eds. Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, IA: Blackwell Publishing, 2007:175.
- Rudman D, Cardiff R, Chouinard L, Goodman D, Küttler K, Marxfeld H, Molinolo A, Treumann S, Yoshizawa K, For the INHAND Mammary, Zymbal’s, Preputial, and Clitoral Gland Working Group. Proliferative and Nonproliferative Lesions of the Rat and Mouse Mammary, Zymbal’s, Preputial, and Clitoral Glands. Toxicologic Pathology 2012: 40:7S-39S.
- Son W and Gopinath C. Early Occurrence of Spontaneous Tumors in CD-1 Mice and Sprague--Dawley Rats. Toxicologic Pathology 2004:32:371-374.
- Yoshizawa K. Specialized Sebaceous Glands. In: Suttie AW, ed. Boorman’s Pathology of the Fisher Rat. San Diego, CA: Academic Press, Inc., 2018: 348-356.
- The Joint Pathology Center (JPC). (2015). Veterinary Systemic Pathology Online (VSPO): I-N11 – Zymbal’s gland adenoma – Zymbal’s gland – rat. Available at: http://www.askjpc.org/vspo/show_page.php?id=355