Signalment:
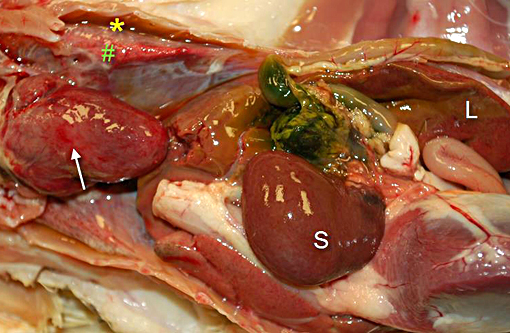
Gross Description:
Histopathologic Description:
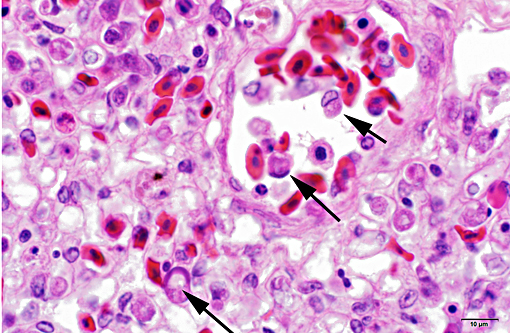
LIVER (Tissue not provided): Within the liver, there is widespread multifocal to coalescent acute hepatic necrosis with mild to marked hemorrhage and hepatic dissociation. Sinusoids and vessels contain numerous red blood cells with eccentric compressed nuclei and round cytoplasmic structures morphologically compatible with protozoal gametocytes (Figure 2). Kupffer cells are enlarged and contain pale orange pigment, cellular debris and occasionally phagocytized erythrocytes.
Morphologic Diagnosis:
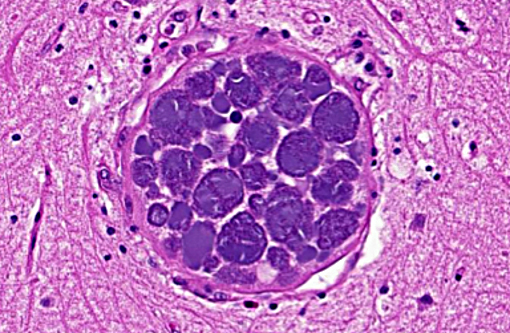
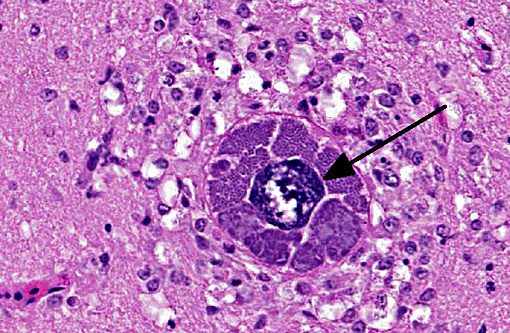
Brain: Mild encephalitis with vasculitis and perivasculitis, mild multifocal necrosis with numerous intraparenchymal megaloschizonts
Liver: Marked acute multifocal to coalescent hepatic necrosis with numerous intraerythrocytic gametocytes and mild erythrophagocytosis
Lab Results:
Condition:
Contributor Comment:
The principal clinical effect of Leukocytozoon spp. infection is intravascular hemolytic anemia, thought to relate to the release of an antierythrocyte factor produced either by the meronts or their host cells rather than the primary mechanical destruction of the red blood cells by the protozoa, since the lowest hematocrit values occur before the parasitemic spike.(4)
In discussions with the referring veterinarian, the black fly population in the area appeared to be very low in early August, but in susceptible ducks, very limited exposure to infected blackflies is sufficient for the intravascular introduction of sufficient sporozoites to cause mortality. The prepatent period for L. simondi infection is approximately two weeks, so this fits well with the length of time from the initial release of the ducks into the outside enclosure and the onset of mortality. The producer has been advised that leukocytozoonosis is one disease that will have to managed if they wish to continue to raise susceptible ducks in Northern Ontario.
JPC Diagnosis:
Conference Comment:
Avian blood parasites include three Haemosporidia genera which are closely related: Plasmodium, Haemoproteus and Leucocytozoon. All are transmitted by a variety of biting insect vectors, which vary depending on the specific parasite, geographic location and distribution. The majority of bird species can become infected, but some species are more susceptible to infection than others (i.e. waterfowl, wild turkeys and penguins are commonly infected, but infection is less common in migratory shorebirds).
In general, Plasmodium and Leucocytozoon species are capable of causing more severe disease, and species of Haemoproteus are considered less pathogenic. Hepatomegaly and splenomegaly may be seen in infection with all three genera, but Plasmodium and Haemoproteus infection results in production of hemozoin pigment from digestion of hemoglobin, which can be seen in the liver, spleen and other organs, and may impart a black or brown appearance to the organs grossly. Leucocytozoon infection does not result in production of hemozoin pigment, so organs will not have the dark discoloration. A presumptive diagnosis can be made based on microscopic examination of a blood film, and the appearance of the parasite in red blood cells (RBC). Leucocytozoon results in the most dramatic change in RBC structure, with enlargement and elongation of RBCs and formation of hornlike extensions at each end of the cells; splitting of the nucleus may also be seen. With Haemoproteus and Plasmodium, less dramatic changes are seen and include slight enlargement of the cells, lateral displacement of the nucleus, hemozoin pigment, and the presence of the small schizonts or gametocyte nuclei.(2) Because the different hemoprotozoa can cause similar lesions, both grossly and histologically, PCR is often necessary to reach a precise etiologic diagnosis.(5)
There are many species of Leucocytozoon, but not all are pathogenic. The species of Leucocytozoon which are pathogenic to wild birds include L. simondi (waterfowl), L. marchouxi (doves and pigeons) and L. toddi (raptors). The species which may cause disease in domestic birds include L. simondi (waterfowl; U.S., Canada, Europe), L. smithi (turkeys; U.S., Canada), L. macleani (chickens; S.E. Asia), L. struthionis (ostriches; S. Africa), L. schoutendeni (chickens; sub-Saharan Africa, S.E. Asia) and L. caulleryi (chickens; south and S.E. Asia).(1)
In Leucocytozoon infection, gross lesions seen in infected birds include splenomegaly, hepatomegaly, tissue pallor and thinning of the blood. Histologically, capillaries may be distended by gametocytes with absence of host tissue reaction. The large megaloschizonts, which are often associated with small vessels, typically elicit a mononuclear inflammatory reaction but the meronts may or may not be associated with an inflammatory response. After the megaloschizonts rupture they may be filled with eosinophilic debris and mononuclear cells. Severe centrilobular hepatic necrosis may be seen along with periportal hepatitis and pigment laden macrophages / Kupffer cells. The enlarged spleen is congested, with loss of normal architecture, and contains large macrophages distended with pigment and cellular debris. Lymphocytic and histiocytic infiltration may be seen in other organs such as the lung and myocardium.(1)
References:
1. Forrester DJ, Greiner EC. Leucocytozoonosis. In: Atkinson CT, Thomas NJ, Hundter DB, eds. Parasitic Diseases of Wild Birds. Ames, Iowa: Blackwell Publishing; 2008:54-107.
2. Friend M, Franson JC, Ciganovich EA. Field manual of wildlife diseases, general field procedures and diseases of birds. Washington DC: U.S. Department of the Interior, U.S. Geological survey; 1999:193-200.
3. Gardiner CH, Fayer R, Dubey JP. Leucocytozoon. In: An Atlas of Protozoan Parasites in Animal Tissue. USDA Agricultural Research Service. Agriculture Handbook # 651; 1988:72.
4. Kocan R. Anemia and Mechanism of Erythrocyte Destruction in Ducks with Acute Leukocytozoon Infections. J Protozol. 1968;15(3):455-462.
5. Schmidt RE, Reavill DR, Phalen DN. Pathology of pet and aviary birds. Ames, IA: Wiley Blackwell; 2015:109-110.