Signalment:
Gross Description:
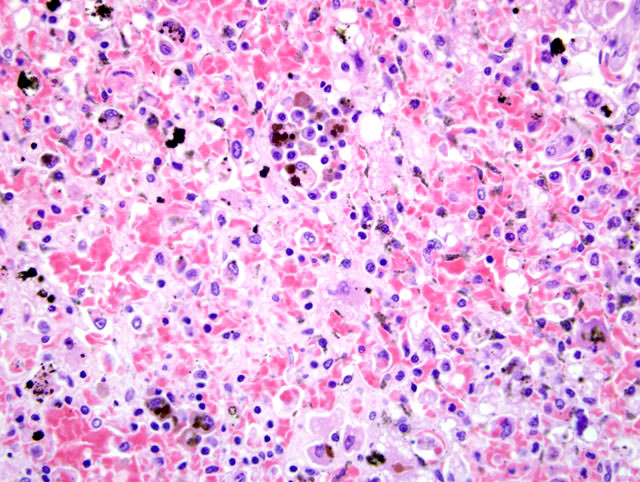
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Xylitol is a 5-carbon sugar alcohol that occurs in small amounts as a natural intermediary during the metabolism of L-xylulose to D-xylulose.(2) It is marketed for human consumption as a sugar substitute. Profound differences exist between species in insulin secretion after administration of xylitol. In humans, rhesus monkeys, rats, and horses, the insulin release is negligible, but in dogs, rabbits, cows, goats, and baboons, insulin levels increase significantly and rapidly.(6) Xylitols effect in cats and ferrets is unknown. In dogs, clinical signs of xylitol ingestion include vomiting, weakness, ataxia, hypoglycemia, hypokalemia, and seizures within 30 to 60 minutes of ingestion.(3)
The pathogenesis of xylitol-induced hepatic necrosis in dogs is not known, although two mechanisms have been proposed.(2) In the first proposed scenario, cellular ATP, ADP, and inorganic phosphorus reserves are depleted by intermediates of xylitol metabolism, leading to loss of membrane integrity and cell death. In the second proposed scenario, reactive oxygen species damage cellular membranes and macromolecules, leading to cell death. These proposed mechanisms are not mutually exclusive, and both, or other as yet unknown, mechanisms may be responsible.(2)
A recent paper describing the histologic lesions in two dogs exposed to xylitol described periacinar to midzonal necrosis (zones III and II) in one sample and centrilobular necrosis (zone III) in the other (2). No zonal distribution was detected in this case, however.
The histologic picture of severe, massive hepatic necrosis is not pathognomonic, and can occur with a variety of hepatic insults, including chemicals (carbon tetrachloride), drugs (carprofen, potentiated sulfonimides), bacterial infections (Clostridium piliformis), and toxins (blue-green algae, Sago Palm seeds, Amanita mushrooms).(1,5,7,8) Anamnesis and clinicopathologic data are needed to prioritize the list of differential diagnoses.
JPC Diagnosis:
Conference Comment:
The livers ability to regenerate is intriguing and unique. The potential for hepatic regeneration depends on the viability of the hepatic stroma. Without stroma, there is no scaffolding for plate organization of new hepatocytes. Normal hepatocytes are in the G0 resting stage of the cell cycle. Damage to the liver results in tumor necrosis factor alpha (TNF-α)-mediated activation of Kupffer cells, which then release IL-6. The effect of IL-6 on hepatocytes allows them to respond to growth factors, such as hepatocyte growth factor (HGF), transforming growth factor-α (TGF-α) and epidermal growth factor. Viable hepatocytes near the area of damage begin replicating. As restoration of the liver mass proceeds, there is increased responsiveness to growth inhibitors TGF-β and activin A. These compounds, which are downregulated during regeneration, suppress hepatocyte replication and induce extracellular matrix .(9)
Participants briefly discussed causes of hypoglycemia which fall into one of six broad categories:(4)
- Excess insulin or insulin analogs
- Reduced hormones that maintain glucose homeostasis
- Reduced hepatic glycogen storage
- Increased glucose usage
- Reduced glucose intake or gluconeogenesis
- Drugs
Based on the signalment, a reasonable differential diagnosis for hypoglycemia in this case would include gramnegative sepsis, malnutrition, paraneoplastic syndrome, insulinoma, or laboratory error. If the serum has prolonged contact with the blood clot in the collection tube, glucose will be depleted because of insulin-independent uptake of glucose by erythrocytes. In addition to marked hypoglycemia, hypophosphatemia, hypokalemia, hypercalcemia and increased levels of alanine aminotransferase (ALT) and aspartate aminotransferase (ASP) have been observed with xylitol intoxication in both experimental and clinical settings.(2,10)
References:
2. Dunayer EK, Gwaltney-Brant SM. Acute hepatic failure and coagulopathy associated with xylitol ingestion in eight dogs. J Am Vet Med Assoc. 2006; 229(7):1113-1117.
3. Dunayer EK. Hypoglycemia following canine ingestion of xylitol-containing gum. Vet Hum Toxicol. 2004;46:87-88.
4. Evans EW, Duncan JR. Proteins, lipids and carbohydrates. In: Latimer KS, Mahaffey EA, Prasse KW, eds. Duncan and Prasses Veterinary Laboratory Medicine: Clinical Pathology. 4th ed. Ames, IA: Blackwell Publishing; 2003:181-187.
5. MacPhail CM, Lappin MR, Meyer DJ, et al. Hepatocellular toxicosis associated with administration of carprofen in 21 dogs. J Am Vet Med Assoc. 1998;212(12):1895-1901.
6. Piscitelli CM, Dunayer EK, Aumann M. Xylitol toxicity in dogs. Compendium 2010. E1-E4. 7. Puschner B, Rose HH, Filigenzi MS. Diagnosis of amanita toxicosis in a dog with acute hepatic necrosis. J Vet Diagn Invest. 2007;19(3):312-7.
8. Senior DF, Sundlof SF, Buergelt CD, et al: Cycad intoxication in the dog. J Am Anim Hosp Assoc. 1985;21:103-109.
9. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed., vol. 2. Philadelphia, PA: Elsevier Ltd; 2007:324-325.
10. Xia Z, He Y, Yu J. Experimental acute toxicity of xylitol in dogs. J Vet Pharmacol Ther. 2009;32:465-469.