CASE IV:
Signalment:
Four-year-old Aberdeen Angus cow, bovine (Bos taurus taurus)
History:
A four-year-old Angus cow presented a clinical history of neurologic signs, which included aggressiveness and isolation from the herd, anorexia, depression and ataxia. Antibiotic treatment (doxycycline) was administered for three days; nonetheless, the clinical condition progressed, and after five days paddling movements, opisthotonus and lateral decubitus were noted. Euthanasia was elected, and the entire head was shipped to the laboratory by the field veterinarian. The referred cow was one of 300 beef cattle that were managed in a cow-calf operation in the state of Rio Grande do Sul, Brazil. The animals were kept in flooded pastures, in a paddock of irrigated rice stubble.
Gross Pathology:
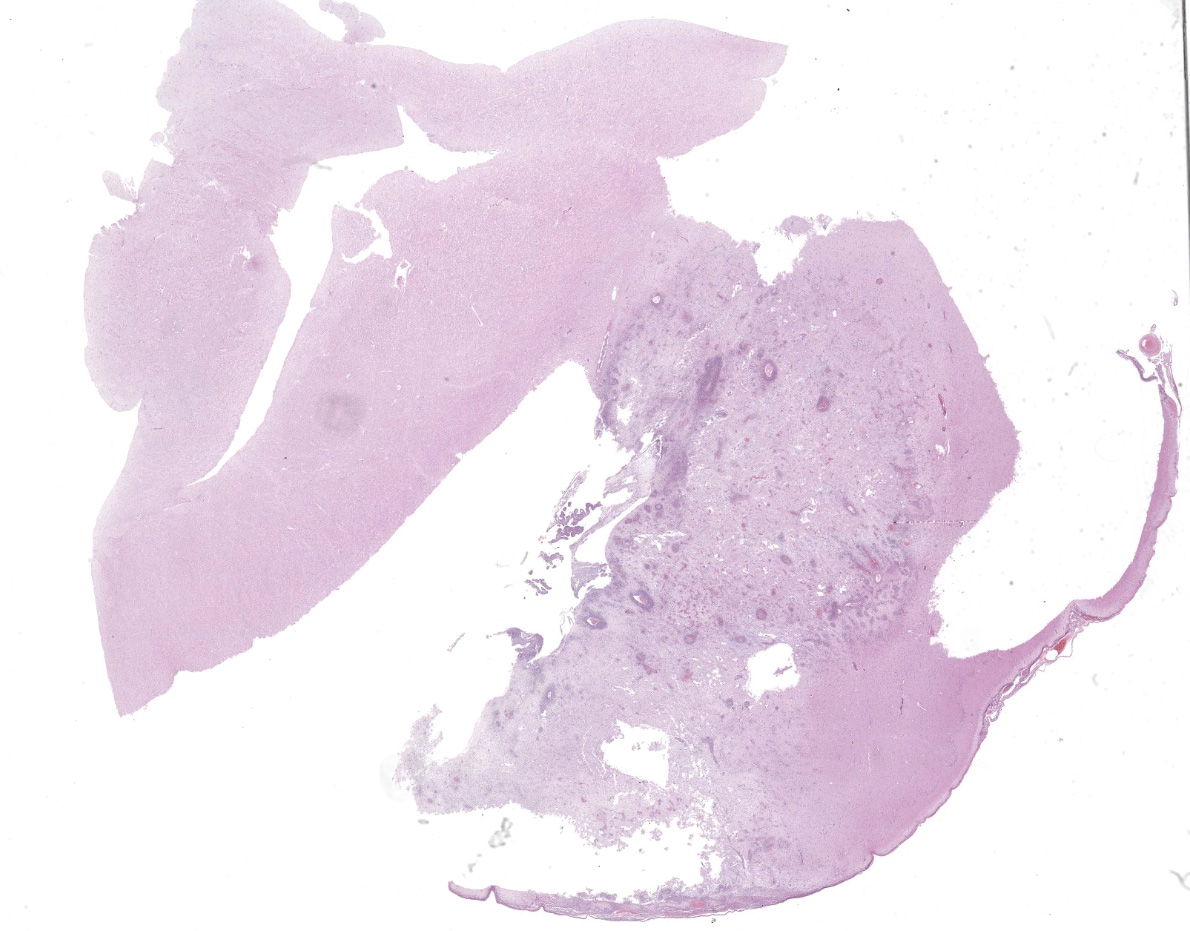
Grossly, the leptomeninges covering the brain, especially the ventral surfaces of the mesencephalon and rhombencephalon presented multifocal, severely thickened dark red areas. Furthermore, the right olfactory bulb showed a soft well-demarcated grayish area of 2.5 cm in diameter. Similar areas, ranging from 0.5 to 2 cm in diameter were noted bilaterally in the piriform lobes, right hippocampus, frontal lobe cortex and fornix. The remaining organs were not available for evaluation.
Laboratory results:
Brain sections were processed for immunohistochemistry (IHC), to search for Acanthamoeba spp., Balamuthia spp. and Naegleria spp., as previously described.1 The amoebae stained positively for Naegleria fowleri through IHC, while immunostaining for Acanthamoeba spp. and Balamuthia spp. was negative.
Microscopic Description:
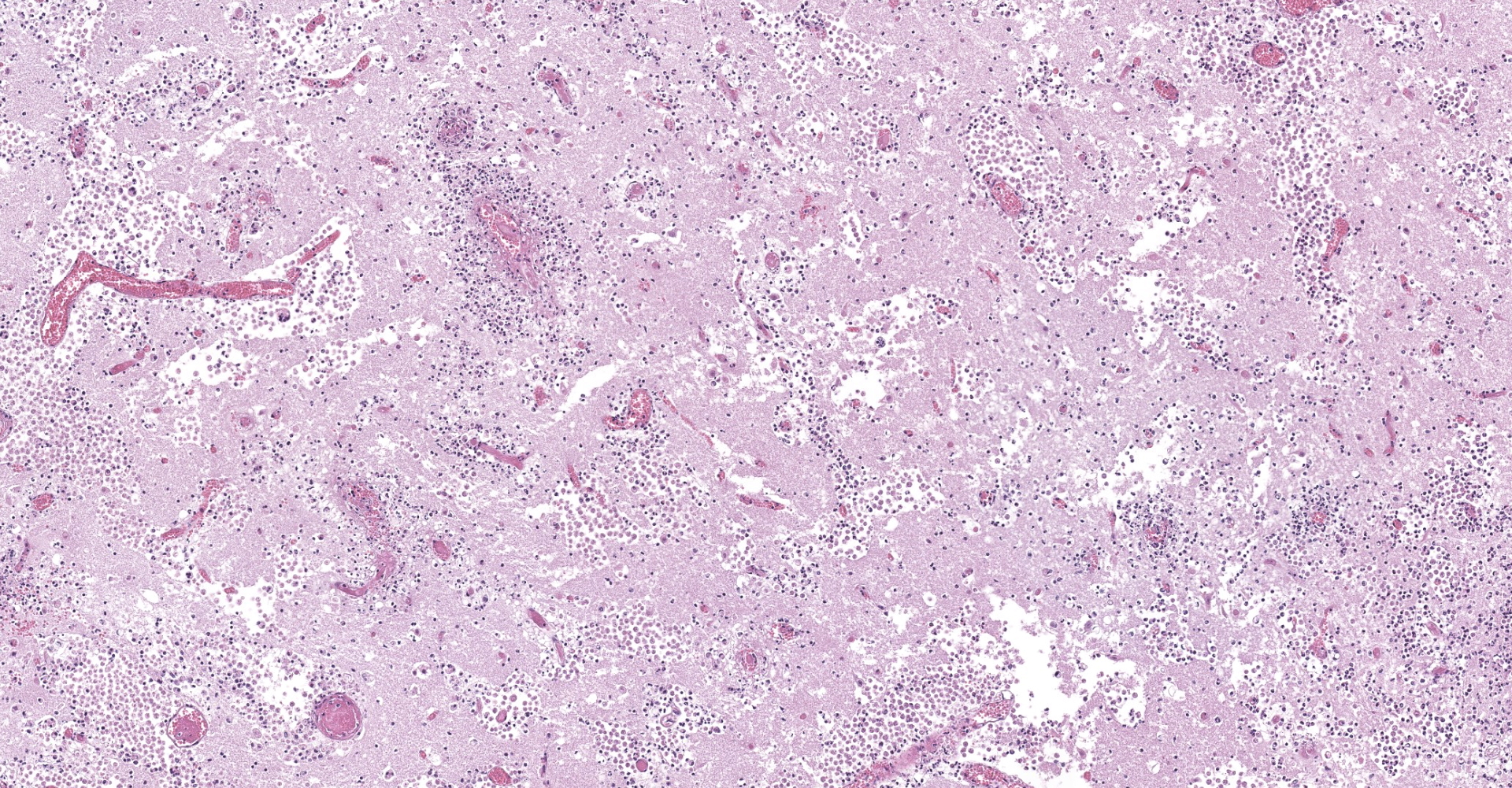
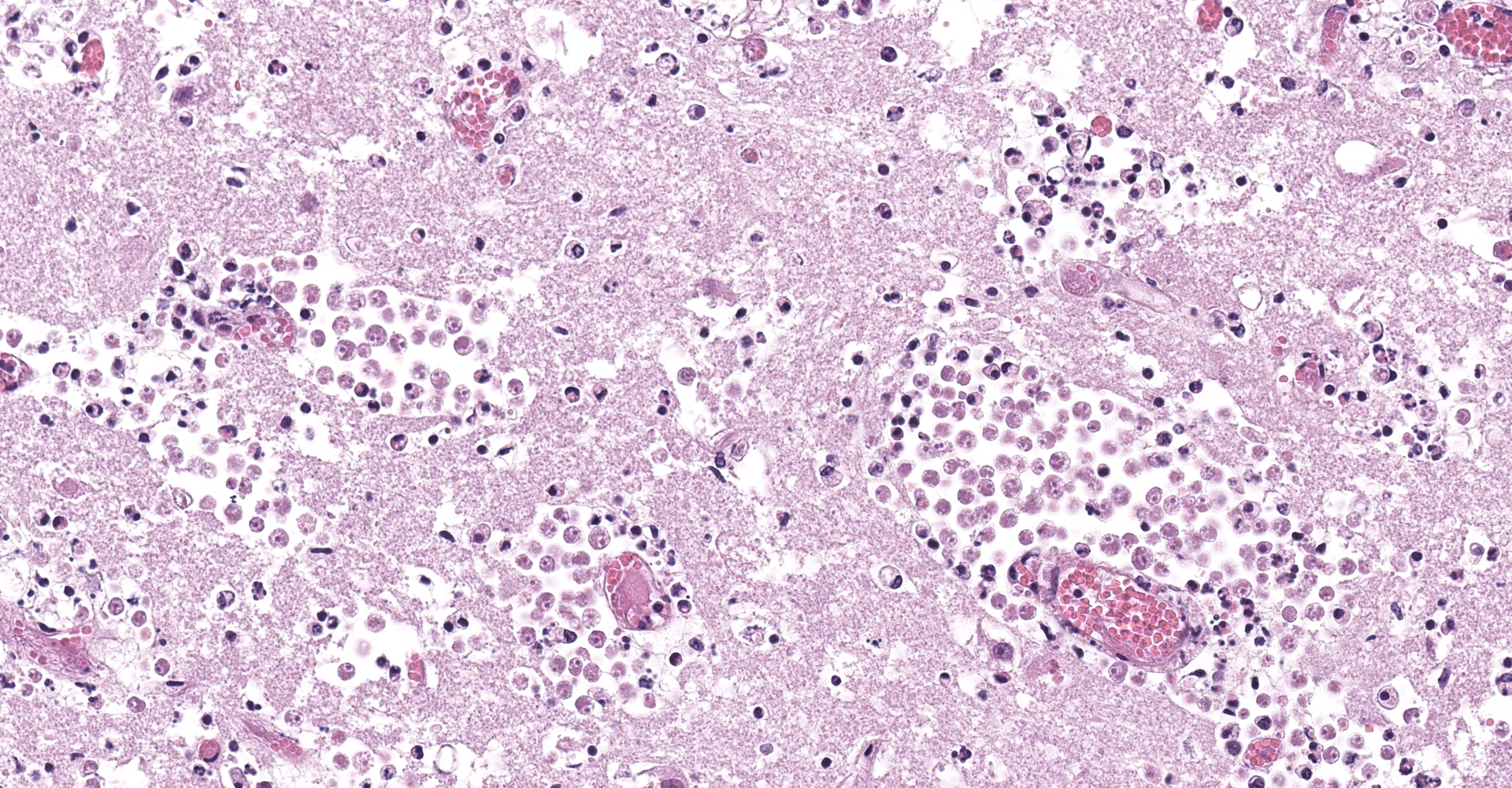
The submitted slides present one section of brain (piriform lobe or mesencephalon), and some slides also display choroid plexus sections. In the brain, there is a focally extensive, well-demarcated area of marked necrosis, surrounded by a halo of severe inflammatory infiltrate of viable and degenerate neutrophils, fewer foamy macrophages (gitter cells), lymphocytes, plasma cells, eosinophils and rare multinucleated giant cells. The referred inflammatory cells are also noted scattered within the necrotic area, mainly in perivascular regions, markedly expanding Virchow-Robin spaces, as well as extending and moderately expanding the adjacent leptomeninges.
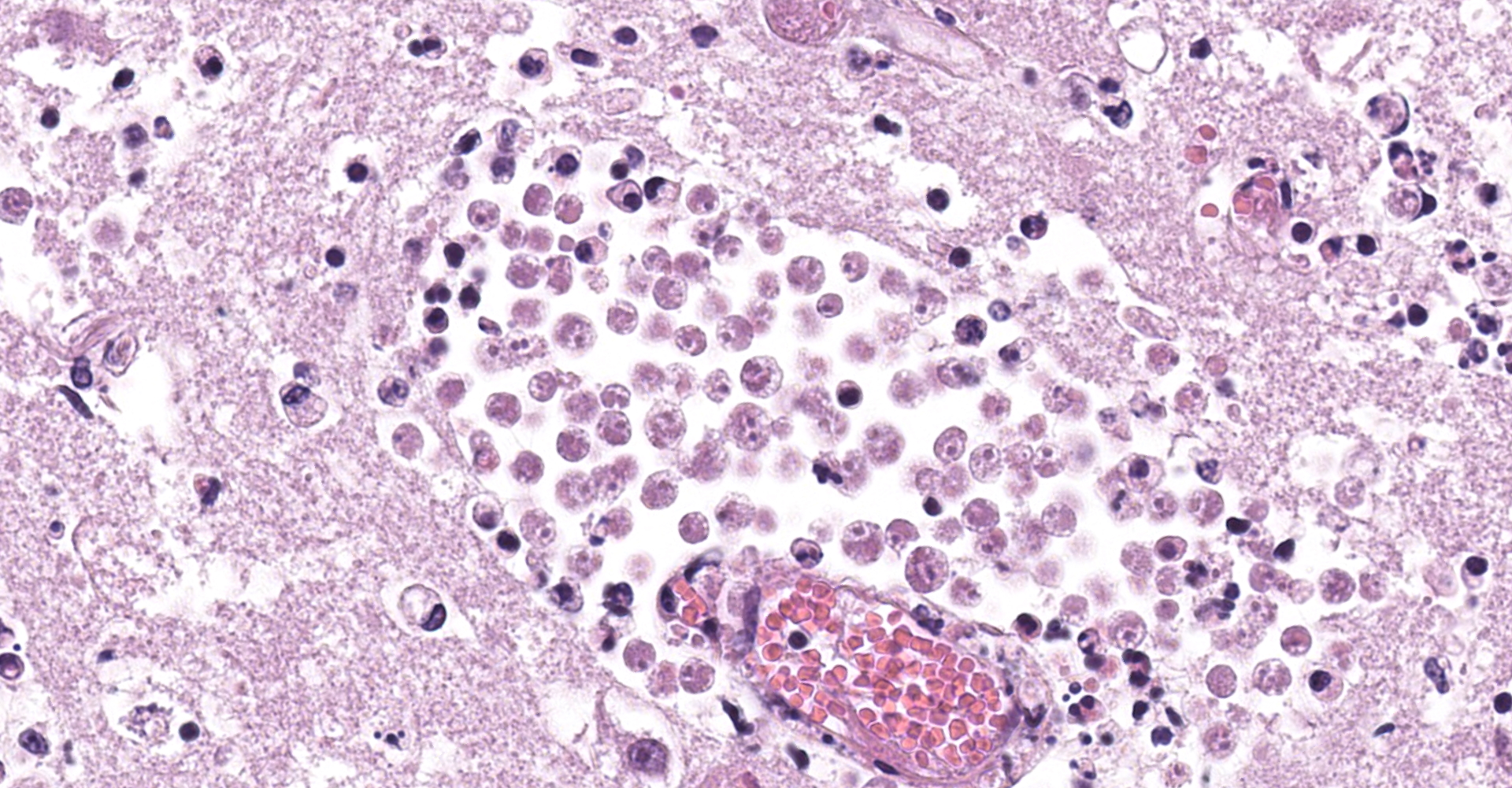
A large number of round to oval amoebic trophozoites measuring around 12 to 15 µm in diameter were seen within the necrotic areas, around and within blood vessels, both in the wall and in the lumen, frequently forming clusters with numerous organisms. These amoebae had abundant granular and pale eosinophilic cytoplasm, eccentric nuclei measuring approximately 3 µm in diameter and had a single 1 to 2 µm hyperchromatic karyosome. The nuclei were surrounded by a clear thin halo.
Furthermore, marked multifocal fibrinoid necrosis and hyalinization of blood vessel walls, characterized by accumulation of amorphous proteinaceous material and inflammatory infiltrate of neutrophils, was noted within the necrotic area. Thrombosis was frequently observed in vessels showing fibrinoid necrosis hyalinization. Moreover, multifocal moderate inflammatory infiltrate comprised predominantly by lymphocytes, plasma cells and fewer neutrophil was noted in the neuropil adjacent to the necrotic area. Similar inflammatory infiltrate was also observed markedly and diffusely expanding the choroid plexus.
In addition to the piriform lobes, similar demarcated lesions were noted affecting both the white and grey matter of the right olfactory bulb, frontal brain cortex, thalamus, mesencephalon, and hippocampus. In the cerebellum, the leptomeninges and Virchow-Robins spaces were diffusely and markedly expanded by a similar inflammatory infiltrate described in the piriform lobe. Furthermore, multifocal areas of severe hemorrhage were seen in the neuropil, as well as multifocal areas of gliosis, which were also noted in the brainstem.
Contributor's Morphologic Diagnoses:
Necrotizing and pyogranulomatous meningoencephalitis, marked, focally extensive, acute, associated with severe multifocal thrombosis, vasculitis and numerous amoebic trophozoites. Lymphohistiocytic and neutrophilic choroid plexitis, acute, marked, diffuse.
Contributor's Comment:
Free-living ameboflagellates of the genera Acanthamoeba, Balamuthia and Naegleria have been associated with disease in people and animals.1,2,4,5,6,7 N. fowleri infection is known to cause a rare entity called primary amoebic meningoencephalitis.3 Most cases of this condition have been reported in people, and the majority of the cases have been described in the USA.8 This protozoon reaches the brain through the olfactory nerve10 causing a highly fatal disease that is restricted to the central nervous system, frequently in immunocompetent individuals.10
N. fowleri is a widely distributed thermophilic free-living amoeba that is commonly found in fresh and hot water.12 The drinking water has been suggested as a potential source of infection in previous reports of N. fowleri infection in cattle.2 In the present case, amoebae were not searched in the environment and sources of water; however, it is plausible to think that the epidemiological characteristics present on the farm, including high temperatures and the use of pastures in seasonally flooded areas, may have contributed to the occurrence of the disease.
After invading the nasal mucosa, N. fowleri is believed to reach the encephalon through the olfactory nerve, consequently causing initial damage to the rhinencephalon.12 The initial lesion is frequently observed as an area of encephalic softening located in the olfactory bulb, and is most commonly unilateral.2 After this initial lesion, N. fowleri tends to spread and cause disseminated lesions in the brain.2
Histologic findings were characterized by severe vascular lesions, including vasculitis and thrombosis. In the present case, these seemed to be caused by direct damage induced by N. fowleri, since great numbers of parasites were observed within the wall of blood vessel, associated with necrosis and neutrophilic inflammation. The referred vascular lesions may have preceded the marked necrotic changes observed, since the latter were interpreted to be the result of infarction, associated with direct damage induced by tissue spread of these agents. Histological visualization of amoebic trophozoites may be challenging.2 Frequently these amoebas may resemble foamy macrophages in areas of suppurative inflammation and necrosis.2 Thus, immunohistochemistry is crucial for establishing the diagnosis, as well as to distinguish these organisms from other amoebas, which are important differential diagnosis in such cases, including Acanthamoeba sp. and Balamuthia mandrillaris.1
Contributing Institution:
Faculdade de Veterinária
Universidade Federal do Rio Grande do Sul
Setor de Patologia Veterinária
JPC Diagnosis:
Cerebrum: Vasculitis and meningoencephalitis, necrotizing, multifocal, severe, with thrombosis and numerous amebic trophozoites.
JPC Comment:
The contributor provides an excellent review of Naeglaria fowleri, one of several mitochondria-bearing, free-living eukaryotic amebae, in addition to Acanthamoeba spp. and Balamuthia mandrillaris, each known to cause infection of the central nervous system (CNS) of humans and other animals.14 Knowledge that free-living amoebae are capable of causing human disease dates back more than 50 years, prior to which time they were regarded as harmless soil organisms or, at most, commensals of mammals.12 In humans, several species of the genus Acanthamoeba cause an insidious and chronic disease, granulomatous amebic encephalitis (GAE), principally in immunocompromised hosts, including those infected with HIV/AIDS. Balamuthia mandrillaris causes GAE in both immunocompromised and immune-competent hosts, mostly in very young or very old individuals. In addition, both etiologies may cause disseminated disease in the lungs, skin, kidneys, and/or uterus. In contrast, N. fowleri causes an acute and fulminating, necrotizing infection of the CNS known as primary amebic meningo-encephalitis (PAM). Children and young adults with history of exposure to warm fresh water are most often affected by PAM.14
As described by the contributor, N. fowleri's route of infection is via direct penetration of the olfactory neuroepithelium via the rhinocerebral route. Upon reaching the olfactory bulb, N. fowleri elicits a significant immune response through activation of the innate immune system, including macrophages and neutrophils. The parasite enters the body in the trophozoite form, which has special structures known as food cups. These structures allow the normally free-living organism to ingest bacteria and fungi, but also tissue of parasitized hosts. In addition, the pathogenicity of N. fowleri is dependent upon the release of cytolytic molecules, including acid hydrolases, phospholipases, neuraminidases, and phospholipolytic enzymes which contribute toward host cell and nerve destruction. The combination of the pathogenicity of N. fowleri and the intense immune response resulting from its presence results in significant nerve damage and subsequent CNS tissue damage, which often results in death.3
Other free-living amoeba may also gain entry to the brain via the rhinocerebral route, however, hematogenous spread following primary lung or skin infection is thought to be more common. An example of this was suggested following a retrospective review of five cases of amoebic meningoencephalitis in gorillas and other old world primates at the San Diego Zoo and San Diego Wild Animal Park between 1965 and 1995 caused by B. mandrillaris. Although the route of infection was unknown, the lack of olfactory lobe involvement was inconsistent with the rhinocerebral route of infection.9
Another significant genus warranting discussion is Entamoeba. Species of this genus inhabit a range of invertebrates and vertebrates, often as commensals in the intestinal tract and less commonly as pathogens. Pathogenic species have an arsenal of virulence factors to enable tissue invasion, including lectins for attachment to intestinal epithelium, mechanisms to cause cytotoxicity of epithelial cells (including induction of apoptosis), secretion of "amoebapore" proteins to lyse cells, a vast array of cysteine proteinases to degrade extracellular matrix, oxygen detoxification mechanisms to allow deep invasion, and finally, various tools to evade the host immune response. E. histolytica, one of the most well-known species of this genus, causes serious disease in primates and is the etiologic agent of human amoebic dysentery, a significant cause of morbidity and mortality in the third world. Another well-known species is E. invadens, which variably affects captive reptiles. In snakes it may be either innocuous or cause severe necrotizing enterohepatitis whereas in turtles it is generally a commensal. Finally, E. ranarum, is rarely associated with intestinal or hepatic disease in captive amphibians.10
Although amebae were typically readily visible with standard HE staining, periodic acid?Schiff reaction (PAS), readily stains the glycogen-laden cytoplasm and can be a useful tool for their identification.10
References:
1. Benterki MS, Ayachi A, Bennoune O, Régoudis E, Pélandakis M. Meningoencephalitis due to the amoeboflagellate Naegleria fowleri in ruminants in Algeria. Parasite 2016; 23(11): 1-4.
2. Daft BM, Visvesvara GS, Read DH, Kinde H, Uzal FA, Manzer MD. Seasonal meningoencephalitis in Holstein cattle caused by Naegleria fowleri. J Vet Diagn Invest 2005; 17:605-609.
3. Grace E, Asbill S, Virga K. Naegleria fowleri: pathogenesis, diagnosis, and treatment options. Antimicrob Agents Chemother. 2015;59(11):6677-6681.
4. Jonckheere JFD. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect Genet Evol 2011; 11: 1520-28.
5. Lozano-Alarcón F, Bradley GA, Houser BS, Visvesvara GS. Primary amebic meningoencephalitis due to Naegleria fowleri in a South American tapir. Vet Pathol 1997; 34(3):239-43.
6. Martinez AJ, Visvesvara GS. Free-living, amphizoic and opportunistic amebas. Brain Pathol 1997; 7:583-598.
7. Morales JA, Chaves AJ, Visvesvara GS, Dubey JP. Naegleria fowleri-associated encephalitis in a cow from Costa Rica. Vet Parasitol 2006; 139:221-223.
8. Pimentel LA, Dantas AFM, Uzal F, Riet-Correa F. Meningoencephalitis caused by Naegleria fowleri in cattle of northeast Brazil. Res Vet Sci 2012; 93:811-812.
9. Rideout BA, Gardiner CH, Stalis IH, Zuba JR, Hadfield T, Visvesvara GS. Fatal infections with Balamuthia mandrillaris (a free-living amoeba) in gorillas and other Old World primates. Vet Pathol. 1997;34(1):15-22.
10. Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Intern J Parasitol 2004; 34:1001-1027.
11. Shilton CM, ?lapeta J, Shine R, Brown GP. Pathology Associated With an Outbreak of Entamoebiasis in Wild Cane Toads (Rhinella marina) in Tropical Australia. Vet Pathol. 2019;56(6):921-931.
12. Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004;34(9):1001-1027.
13. Siddiqui R, Ali IKM, Cope JR, Khan NA. Biology and pathogenesis of Naegleria fowleri. Acta Trop 2016; 164: 375-394.
14. Visvesvara GS. Infections with free-living amebae. Handb Clin Neurol. 2013;114:153-168.