CASE IV: 487/0821 (JPC 4165420)
Signalment:
45-day-old, female, Canary Black Pig (Sus scrofa domesticus)
History:
One female piglet, from a farm with 28 breeders (25 sows and 3 boards), had been found outside the farm wandering near rubbish dumps. Two days later, it exhibited lethargy, dehydration, hypothermia, and cachexia, along with vomit and mucopurulent nasal discharge. The animal died within a day after the signs were noticed. No specific treatment was administered other than fluids and electrolytes. No other pigs from the farm showed any clinical signs.
The animal was submitted to our diagnostic laboratory for a complete necropsy.
Gross Pathology:
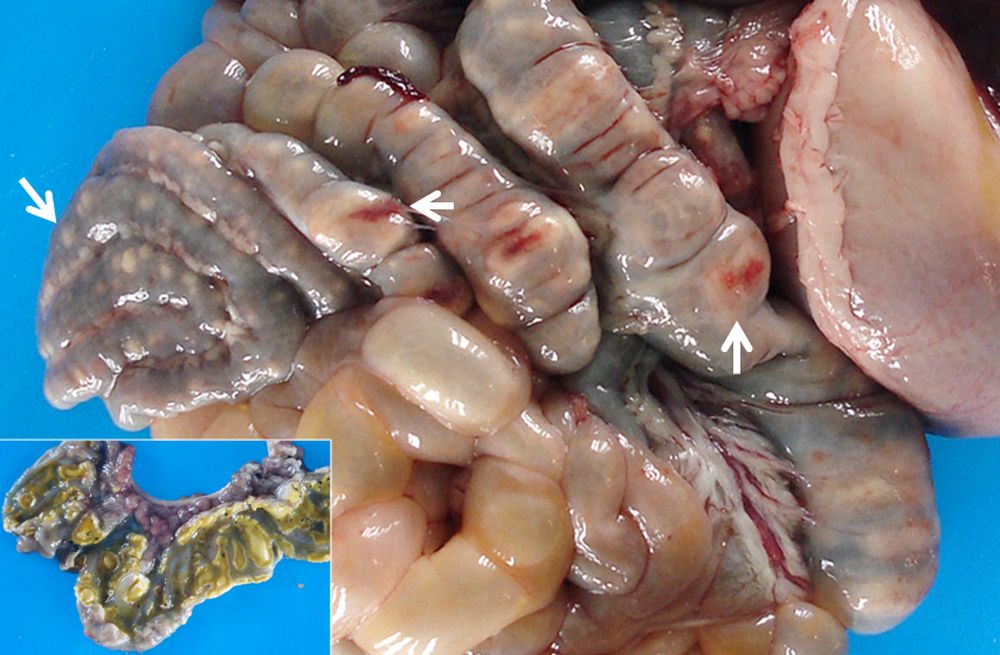
The most remarkable macroscopic lesions were observed in the caecum, colon, liver, and palatine tonsils. Multiple well-demarcated white nodules were observed on the serosa of the spiral colon. The mucosa of both caecum and colon showed numerous multifocal to coalescent well-demarcated ulcers, covered with fibrinosuppurative (diphteroid) membranes. In the liver, on the visceral surface of the caudate lobe, a locally extensive 5 cm-wide area of necrosis was observed. On cut surface, the necrotic lesion extended deep into the parenchyma. The palatine tonsils had focal and bilateral areas of necrosis and congestion. Other gross findings included serous abdominal effusion, and enlarged and congested mesenteric lymph nodes.
Laboratory Results:
Bacterial analyses including typing (Reference Laboratory, Algete-Madrid, Spain) identified Salmonella enterica subspecies enterica Serotype: Enteritidis 9,12: g.m. from samples of large intestine, liver and mesenteric lymph nodes.
Microscopic Description:
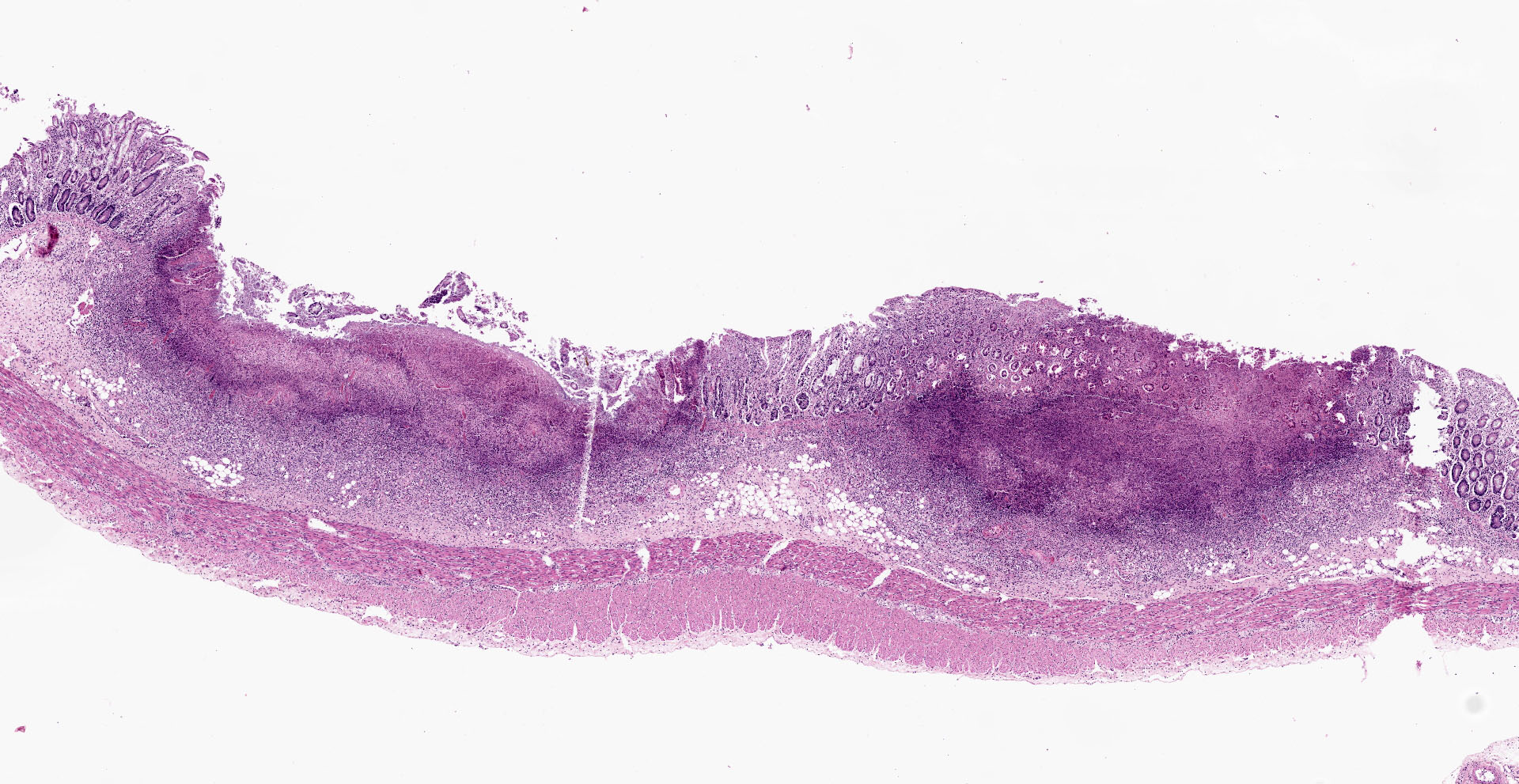
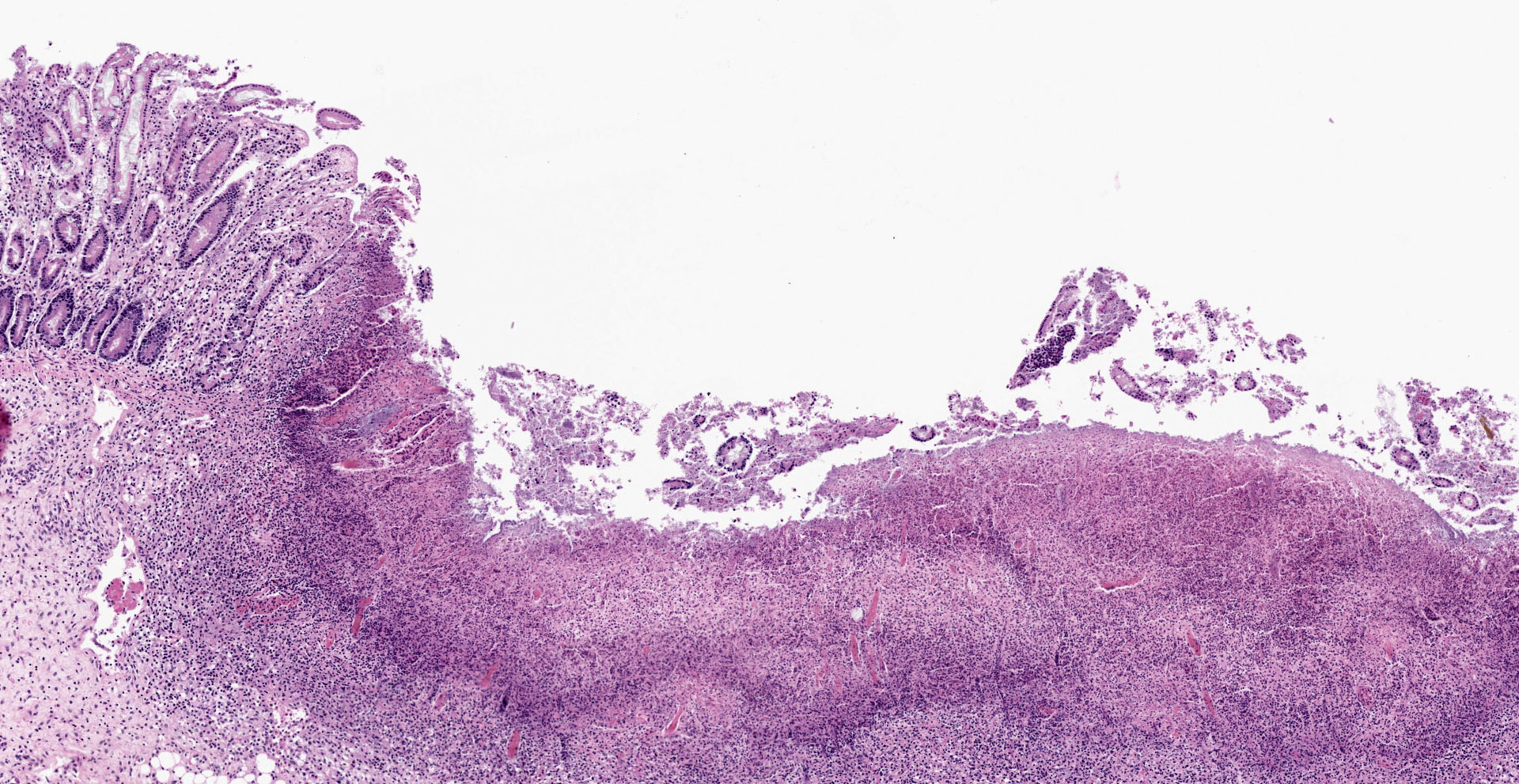
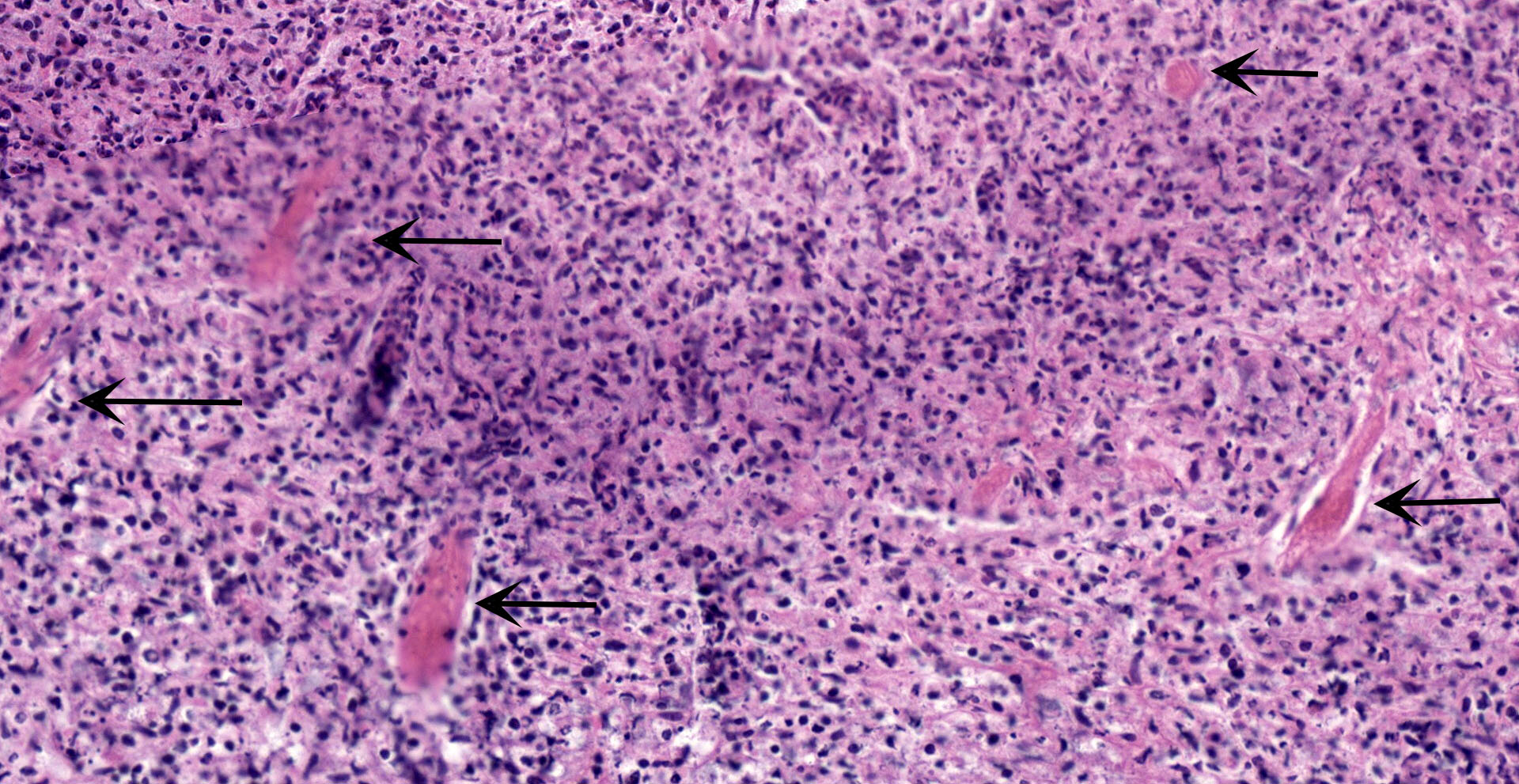
Colon: affecting the full thickness of the mucosa and extending into the submucosa there are large, segmental, well-demarcated areas of necrosis covered with fibrin (button ulcers). These areas are characterized by loss of differential staining, loss of tissue architecture and replacement by abundant eosinophilic amorphous material, cellular debris, fibrin and degenerated neutrophils, admixed with abundant short rod bacteria. The underlying submucosa is severely infiltrated by viable neutrophils, macrophages and cellular debris, and expanded by edema. Few blood vessels are occluded by fibrin thrombi, together with occasional small number of short rod bacteria. Vascular walls show karyorexis/karyolisis and loss of cellular detail and are replaced by eosinophilic amorphous material and debris (fibrinoid necrosis). Moderate numbers of neutrophils and macrophages, with rare lymphocytes, infiltrate vascular walls and occasionally fully occlude the lumen (vasculitis). There is marked hypertrophy and hyperplasia of submucosal fibroblasts with plump nuclei (reactive). Multifocally, the same inflammatory infiltrate extends into the muscular layer, dissecting the myofibers.
Contributor's Morphologic Diagnoses: Colitis, necrotizing, fibrinosuppurative (diphteroid), multifocal, severe, subacute, with vasculitis, thrombosis and intralesional colonies of short rod bacteria.
Contributor's Comment:
In addition to the lesions observed in the colon and caecum, other significant histological findings were hepatitis, necrotizing and lymphohistiocytic, tonsilitis necrotizing and neutrophilic, pneumonia, bronchointersticial, lymphohistiocytic, and microthrombosis in the spleen, lymph nodes, brain and leptomeninges. Gram stain highlighted intralesional positive and, to a lesser extent, negative rod-shaped bacteria in palatine tonsils, and small to moderate numbers of negative bacteria in the liver.
The Canary Black Pig (Cochino Negro Canario) is a breed exclusive to the territory of the Canary Islands archipelago. It seems to come from North African pigs, mixed with breeds introduced during the Spanish and British occupation of the islands in the 15th century. The breed was officially recognized once included in the Official Register of Spanish Livestock Breeds published in the Real Decreto (Royal Decree) 1682/19974 and it is currently under a Special Protection Status due to the reduced number of individuals and high risk of extinction. However, since the 80s, the population has been increasing, thanks to the numerous initiatives taken by both local authorities and a group of farmers who decided to introduce the breed in their facilities.6
Salmonellosis is known to be one of the main causes of food-borne enteric disease in humans worldwide. Salmonella Enteritidis and S. Typhimurium are the most commonly reported serovars associated with human cases. These zoonotic strains are carried by both livestock and wild animals, with avian species and pigs being the main reservoirs. In general, infection in birds is caused by serovar Enteritidis, while porcine infections are usually due to serovar Typhimurium or serovar Derby. However, in several European countries, S. Enteritidis has also been reported, in both domestic and wild suids.2,5,8,9 Thus, in swine, salmonella infections are of concern for two main reasons: clinical disease in animals (salmonellosis) and the threat to human health by means of contaminate pork products with a broad range of serotypes.3
Fecal?oral transmission is the most common route of transmission of virulent salmonellae. Transmission can occur from pig to pig, contaminated environment to pig, or dam to offspring. Tonsils, and thus oropharyngeal secretions, become rapidly contaminated, favouring nose-to nose transmission. Aerosolized secretions, faeces, and contaminated dust particles are potential sources for short-distance aerosol transmission as well.3 Over 200 virulence factors have been associated with salmonellae but only few have been completely characterized. Generally, these factors are involved in adhesion, invasion, cytotoxicity, and resistance to intracellular killing. The ability to invade is a requirement for pathogenesis and depends on a serotype-specific plasmid coding for virulence factors.3 Many epithelial cell types as enterocytes, goblet cells or M cells in the jejunum and ileum may be invaded. Invasion of M cells seems to be the preferred route due to the shorter glycocalyx that coats these cells.3 Attachment of the bacteria to epithelial receptors triggers microfilament-controlled uptake, vacuole formation, vacuole transport through the cell cytoplasm, and entry into the lamina propria via exocytosis through the basement membrane.
During the invasion process, there is selective induction of synthesis of new proteins that enhance intracellular survival within macrophages and neutrophils in the lamina propria.3 Salmonella uses mucosal macrophages and dendritic cells to spread systemically, reaching, for instance, liver and lymph nodes, which are common sites of secondary infection (septicemic form). Salmonellae produce disease via enterotoxins, cytotoxins (verotoxins), and endotoxins causing microvascular thrombosis and endothelial necrosis in the submucosa and lamina propria. Mucosal ischemia is most likely the result of microvascular thrombosis and the action of enzymes and mediators released during the inflammatory process.10 The systemic signs and lesions of septicemic salmonellosis, are commonly attributed to the endotoxemia that follows bacterial dissemination.
The number of potential sources of Salmonella infection in a swine population is virtually endless, and many stressors can be involved (e.g. overcrowding, transportation, concurrent diseases, feed changes, parturition and antibiotic treatments).7,10 Although none of those were identified in this specific case, individual susceptibility and the age of the animal could be considered predisposing factors, as disease is usually more common and severe in young individuals.10 In the present case, it is possible that contaminated material at the reported small rubbish dump was the source of infection. The fact that all the other animals at the farm tested negative for Salmonella supports this hypothesis. Housing of pigs in facilities other than total confinement is a factor associated with infection.3
Contributing Institution:
Unit of Veterinary Histology and Pathology.
University Institute of Animal Health and Food Safety (IUSA), Veterinary
School, University of Las Palmas de Gran Canaria.
https://hcv.ulpgc.es/web2/?page_id=3601
JPC
Diagnosis:
Colon: Colitis, ulcerative, multifocal, severe, with vasculitis and thrombosis.
JPC Comment:
With the notable exception of the host adapted Salmonella Cholerasuis, the majority of porcine Salmonella infections result in self-limiting diarrhea or are asymptomatic. As mentioned by the contributor, the majority of porcine infections are caused by Salmonella Typhimurium, which has been linked to numerous outbreaks of human salmonellosis directly related to the consumption of pork products.1
As noted by the contributor, the jejunum and ileum are believed to be the primary sites of Salmonella invasion following ingestion. Utilizing previously described mechanisms, the organism breaches the mucosal epithelium, enters the lamina propria, undergoes leukocyte trafficking to the subepithelial dome of Peyer's patches and then quickly spreads to mesenteric lymph nodes where in most cases its dissemination is halted by cellular and inflammatory responses. However, it is believed high numbers of infected pigs become carriers with latent organisms remaining in the tonsils and mesenteric lymph nodes, which presents a challenge for control of the disease within a herd.1
A recent study assessed the persistence of Salmonella Typhimurium infection of weaned pigs using using Taqman qPCR to quantify Salmonella in the contents of the ileum, cecum, colon, and feces and while also performing histologic examination of tissues. Mucosal epithelium was severely damaged 1 day post infection (dpi) and 2 dpi with large quantities of Salmonella detected in the ileum, cecum, and colon early in the infection, likely bearing resemblance to lesions seen in this case. After 6dpi, signs of recovery with progressive epithelial restoration, reduction of inflammatory infiltrate, and elimination of Salmonella from the mucosa were noted. Although clinical recovery correlated with decreased shedding, Salmonella continued to be passed four weeks post infection. In addition, the bacteria were identified within the mesenteric lymph nodes up to 30dpi, the end point of the study. This further supports the latent risk of reinfection of pigs as well as potential food chain contamination.1
Interestingly, recent studies have demonstrated Salmonella infects and survives not only within macrophages but also fibroblasts, which both may serve as Trojan horses, shielding the organism from immune surveillance until conditions are favorable for reinfection.1
Although Salmonella Enteritidis was isolated in this case, the lesions observed are essentially identical to those caused by classical swine fever virus (hog cholera), an endotheliotropic Pestivirus. This virus predominantly affects the immune system, vascular endothelium, and epitheial cells. CSFV replicates within lymphoid tissue macrophages and causes lymphocytolysis via expression of cytokines such as TNF-α. Lesions consistent with this phenomenon in the submitted tissue sections include GALT abscessation and lymphoid depletion, although these findings in this case are likely secondary to circulation endotoxin. Epithelial damage due to CSFV is also as the result of macrophage derived cytokines. Damage to the endothelial cells can ultimately result in disseminated intravascular coagulopathy and hemorrhage.7
The moderator discussed additional etiologies that may cause similar lesions of the colonic mucosa in swine, including classical swine fever virus, Brachyspira hyodysenteriae and B. pilosicoli, Lawsonia intracellularis, and in very young piglets, Clostridium difficile.
Additional sections stained with Warthin-Starry revealed numerous argyrophilic spirochetes associated with the superficial aspect of mucosal epithelial cells. It is possible these represent a coinfection with Brachyspira spp. in addition to S. Enteritidis.
References:
1. Bellido-Carreras N, Argüello H, Zaldívar-López S, et al. Salmonella Typhimurium Infection Along the Porcine Gastrointestinal Tract and Associated Lymphoid Tissues. Vet Pathol. 2019;56(5):681-690.
2. García-Feliz C, Collazos JA, Carvajal A, Vidal AB, Aladueña A, Ramiro R, De La Fuente M, Echeita MA, Rubio P. Salmonella enterica infections in Spanish swine fattening units. Zoonoses Public Health. 2007;54:294?300.
3. Griffith RW, Carlson SA, Krull AC. Salmonellosis. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, eds. Diseases of Swine. 11th ed. New Jersey: Wiley-Blackwell; 2019:912 ? 925.
4. Real Decreto 1682/1997. Official Register of Spanish Livestock Breeds http://www.boe.es/boe/dias/1997/11/21/pdfs/A34205-34207.pdf
5. Report of the Task Force on Zoonoses Data Collection on the Analysis of the baseline survey on the prevalence of Salmonella in slaughter pigs in the EU 2006 2007, Part B. EFSA J. 2008;206:1?111.
6. Robert A, Zamorano MJ, Ginés R, Argüello A, Delgado JV, López JL. Origin and standing of Canary Black Pig. Archivos de Zootecnia. 2000;49:291?296.
7. Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG,eds. Pathology of Domestic Animals. 6th ed. Missouri: Elsevier; 2016:1?257.
8. Volf J, Stepanova H, Matiasovic J, Kyrova K, Sisak F, Havlickova H, Leva L, Faldyna M, Rychlik I. Salmonella enterica serovar Typhimurium and Enteritidis infection of pigs and cytokine signalling in palatine tonsils. Vet Microbiol. 2012;156:127?135.
9. Wacheck S, Fredriksson-Ahomaa M, König M, Stolle A, Stephan R. Wild boars as an important reservoir for foodborne pathogens. Foodborne Pathog Dis. 2010;7(3):307?312.
10. Zachary JF. Mechanisms of Microbial Infections. In: JF Zachary Ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, Missouri: Elsevier;2017:132 ? 241.