CASE 1: RUSVM CASE 1 (JPC 4085383-00)
Signalment:
8 years, spayed female, mixed, Canis familiaris, Canine
History:
Accidentally run over by owner, presented with multiple trauma, euthanized due to poor prognosis.
Gross Pathology:
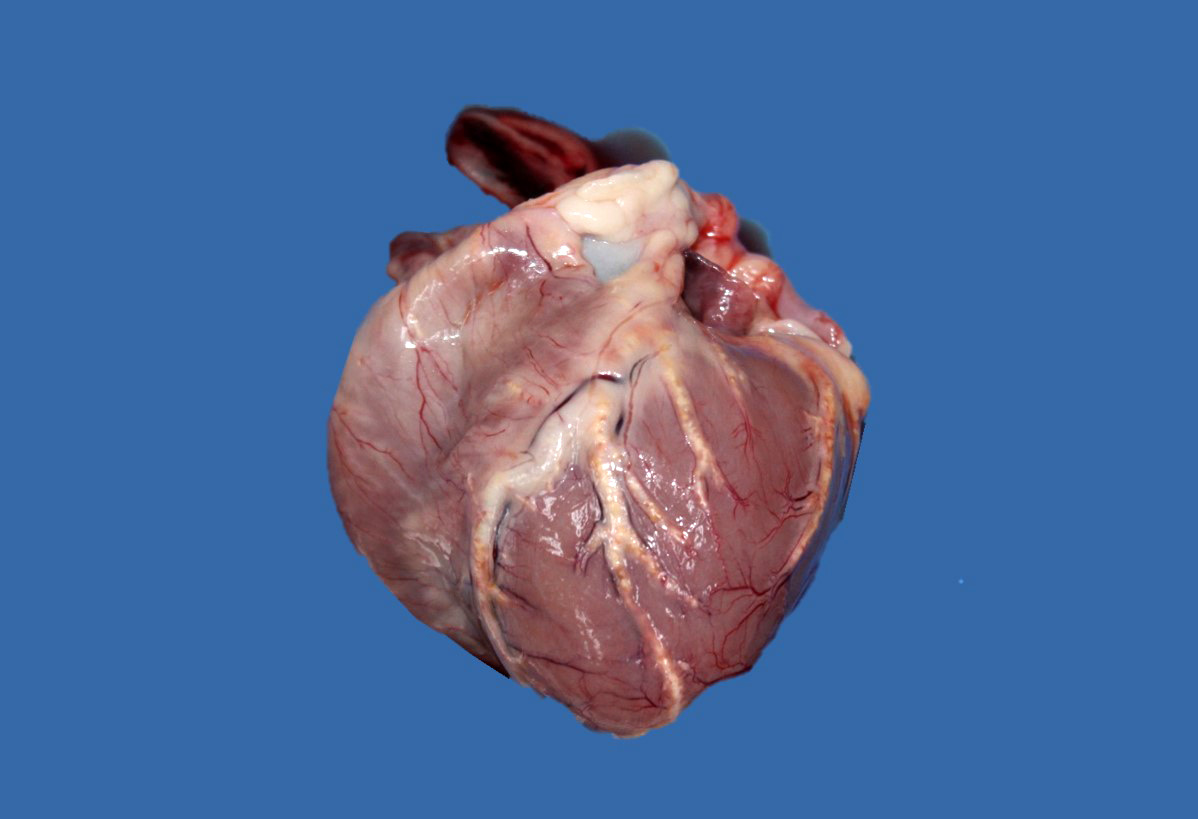
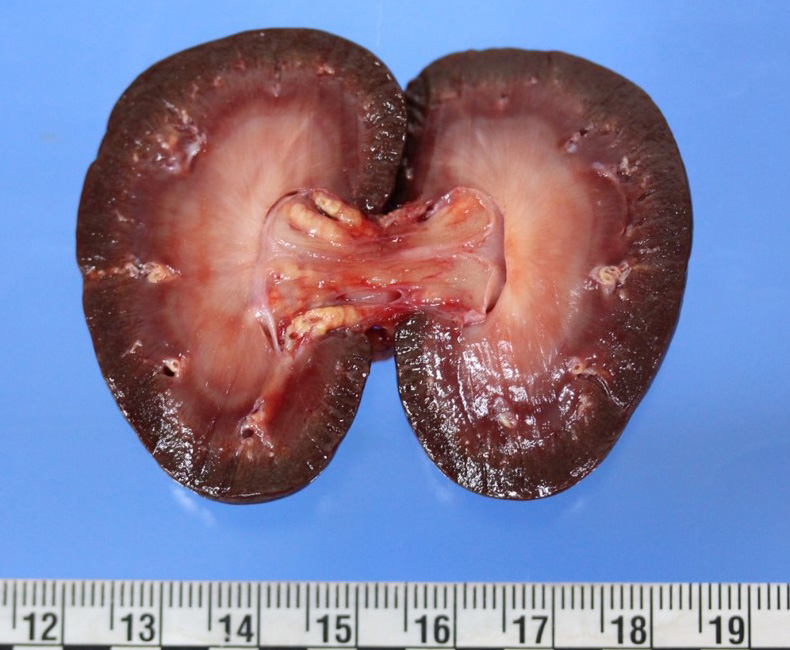
Upon internal examination numerous arteries were prominent, irregularly thickened, tortuous, white-yellowish and hard to cut with a gritty consistency (mineralization). Most notably involved were the coronary, dorsal vertebral, gastric, carotid, as well as renal arteries. The descending aorta had similar changes. Some smaller size arteries within the kidneys were also affected. Interestingly, the arteries in the brain presented no gross or microscopic lesions. Marked bilateral symmetrical atrophy of the thyroid glands was observed.
Laboratory results:
N/A
Microscopic description:
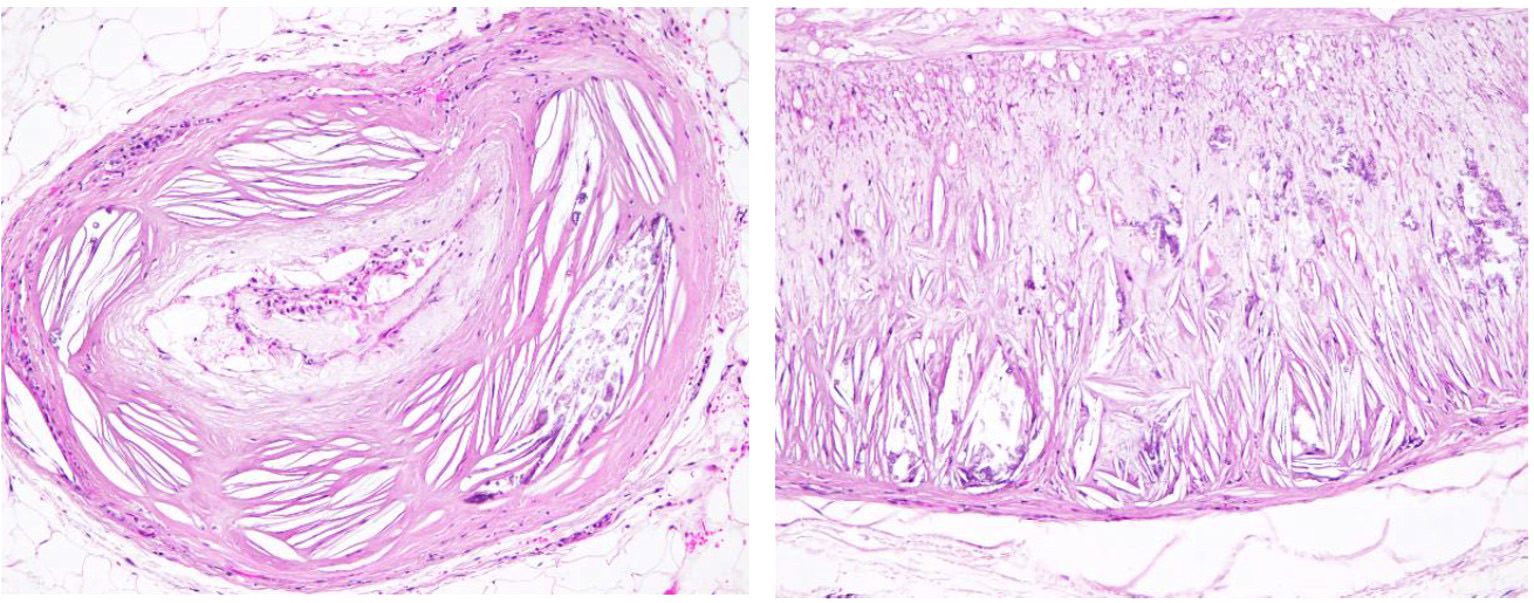
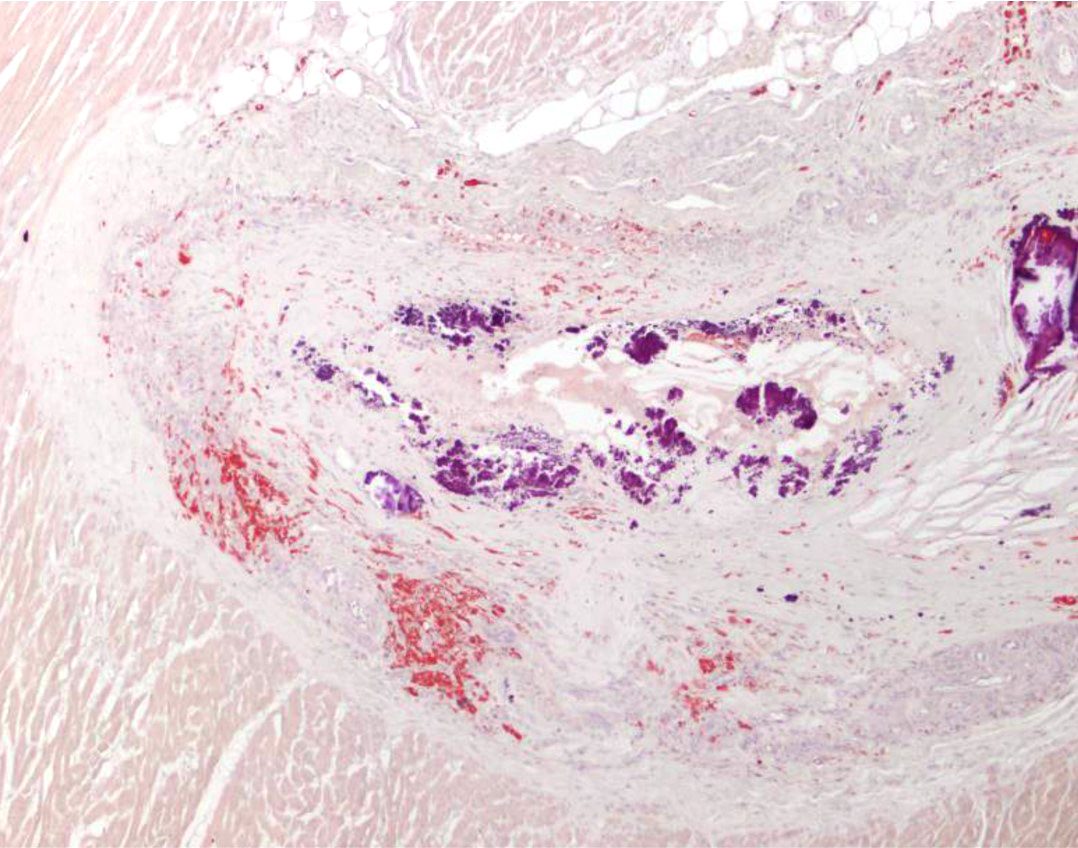
The tunica media and intima of most of the medium and large size renal arteries were irregularly infiltrated and expanded by a combination of fibrosis, acicular cholesterol cleft depositions, mineralization and inflammatory cell infiltration mainly by macrophages, lymphocytes and plasma cells. In some areas these changes were transmural and foamy lipid-containing cells (macrophages and/or smooth muscle cells) were commonly observed.
A large re-canalized thrombus partially occluding most of the lumen of a branch of the renal artery was observed in the right kidney (not submitted). The thrombus was formed of laminar areas of fibrin, lipids, cell debris, hemorrhage, viable and degenerate neutrophils, foamy macrophages, lymphocytes and plasma cells.
Multifocally within the cortex renal tubules exhibited vacuolated and often attenuated epithelium. Pyknotic nuclei and intratubular cell debris (degeneration and necrosis) were occasionally seen. Evidence of tubular regeneration (basophilic and hypertrophic tubular epithelium) was present in some areas. Multifocally within the tubular epithelium finely granular golden-brown intracytoplasmic pigment was observed; this pigment stained negative with Pearl's stain and was interpreted as bile pigment. Microscopical evidence of chronic liver disease was detected in this dog (no liver section submitted). In addition, intracytoplasmic accumulations of a finely granular eosinophilic proteinaceous material (compatible with hyaline droplets) were present within proximal tubules. Multifocally, tubules were moderately ectatic, lined by attenuated epithelium, and were occasionally filled with varying amounts proteinaceous material (protein casts).
The glomeruli were slightly hypercellular and appeared either expanded by mildly thickened capillary loops or had a mildly thickened Bowman's capsule with hypertrophy of the parietal epithelium and occasional synechia formation. Some glomeruli had ectatic urinary spaces due to the presence of variable amounts of proteinaceous material leading to atrophy, loss or fibrosis of the glomerular tuft.
Some degree of autolysis was also observed.
Contributor's morphologic diagnosis:
1. Large and medium size arteries: atherosclerosis, diffuse, severe with cholesterol clefts and mineralization.
2. Nephritis, interstitial, lymphoplasmacytic, chronic, mild, multifocal.
3. Tubular degeneration, necrosis and regeneration with bile pigment and hyalinosis, moderate, multifocal.
4. Membranoproliferative glomerulonephritis, moderate with occasional glomerulosclerosis and atrophy, multifocal.
Contributor's comment:
Atherosclerosis has little practical importance in domestic animals, except as a model of the human disease. Animals have different susceptibility to atherosclerosis with rabbits, chickens, and pigs being more sensitive, and dogs, cats, cattle, goats, and rats being traditionally considered atheroresistant. The best large-animal models of human atherosclerosis are swine and nonhuman primates.3
Of the domestic animals, the deposition of cholesterol and other lipids in the arteries in more than trace amounts occurs only in dogs and is almost always a consequence of hypercholesterolemia associated with hypothyroidism or diabetes mellitus. Miniature Schnauzers are predisposed to atherosclerosis maybe as a result of idiopathic hyperlipoproteinemia.3
The deposition of lipids in dogs begins in the middle and outer layers of the media and occurs more extensively in the small muscular arteries than in the large elastic ones. The media may be greatly increased in thickness by the accumulation of lipid, most of which is in foam cells, but some is present in identifiable smooth muscle cells of the media or free in the interstitium as droplets or crystals. The deposition of lipid in the internal layers of the media leads to disruption of the internal elastic lamina and involvement of the intima. There are no changes associated with the veins.3
Even though gross lesions in several major organs such as heart, brain, or kidney can be impressive, clinical consequences of atherosclerosis are infrequent in dogs. Nevertheless, rupture of atheromatous plaques into the vascular lumen with subsequent thrombosis or even widespread lipid embolism have been observed.3
There are a few differences in the morphology of atherosclerotic lesions between dogs and in humans; in dogs some degree of lipid deposition is observed in the intima, but the primary location is in the media and adventitia of atherosclerotic arteries, whereas in humans, lipid is present primarily in the intima.3
Contributing Institution:
Ross University School of Veterinary Medicine
Department of Biomedical Sciences
JPC diagnosis:
1. Kidney, renal, arcuate and sublobular arteries: Atherosclerosis, diffuse, severe.
2. Kidney: Nephritis, interstitial, lymphoplasmacytic, diffuse, mild.
JPC comment:
While atherosclerosis typically results in fewer cases of overt disease in veterinary species, it is widespread in its reported cases. Apes, elephants, perissodactyls, captive and free ranging bottlenose dolphins, emu, penguins, captive and free ranging birds of prey, psittacines, finches, hornbills, rhamphastids, and a variety of lizard species.5
The pathogenesis of atherosclerosis is multifactorial, with most research performed in humans. The generation of atherosclerotic plaques involves interaction between endothelium, smooth muscle cells, platelets, T lymphocytes, and monocytes. When low-density lipoprotein cholesterols are oxidized, they may home to and damage endothelium. Monocytes arrive at the site and attempt to phagocytose the cholesterol from the tunica intima, resulting in foam cells. The surplus of oxidize LDL induces additional metabolic changes that create a procoagulant environment. Platelets may aggregate, and form thrombi.2
Using information gained from experimental models, it has been shown that CD4+ Th1 cells and natural killer T cells (NK-T) have pro-atherogenic properties, while Treg cells are anti-atherogenic. While the specific roles of some subtypes have yet to be elucidated (Th2, Th9, Th17, Th22, TFH, CD8+, ?? ? cells), we know the microenvironment can force Treg lymphocytes to convert into proinflammatory subtypes Th1, Th17, or TFH. Recruitment of T lymphocytes to the atherosclerotic plaque occurs though the chemokines and chemokine receptors CCR5, CXCR3, and CXCR6. The current body of research suggests atherosclerosis is a chronic inflammatory disease with an autoimmune component, but further research is needed to identify all relevant self-antigens.4
The moderator commented on the importance of animal models in human research of atherosclerosis. An ApoE-knockout mouse model has been used to monitor the progression of this disease in the hopes that the research may improve human outcomes. Apo-E knockout mice fed a high fat diet developed characteristic atherosclerotic lesions most suitable for comparison in the brachiocephalic artery, and in the coronary artery to a lesser extent.1
References:
- Li Y, Zhang CG, Wang X-H, Liu DH. Progression of atherosclerosis in ApoE-knockout mice fed on a high-fat diet. Eur Rev Med Pharmacol Sci. 2016;20(18):3863-3867.
- Miller AD, Zachary JF. Nervous System. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease, 6th Ed. St. Louis, MO:Elsevier. 2017:856-857.
- Robinson WF and Robinson NA: Cardiovascular system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 6th ed., Vol. 3, pp. 57-59. Elsevier Saunders, St Lois, Missouri, 2015.
- Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17(7):387-401.
- Terio KA, McAloose D, St. Leger J, eds. Pathology of Wildlife and Zoo Animals. San Diego, CA: Elsevier. 2018.