CASE 4: D18-10210 (4135869-00)
Signalment:
6-month-old, male intact inland bearded dragon (Pogona vitticeps)
History:
The animal had a 5-d course of anorexia and lethargy with terminal right head tilt. The animal was fed a daily diet of 10?15 superworms (commercially purchased), green turnips, and collard greens. The animal died 1 d after the owner noted that the beard of the animal had turned black.
Gross Pathology:
The animal was in a good nutritional state, weighing 145 g. The pericardial sac was markedly dilated and filled with clotted and unclotted blood. Fibrinous exudate adhered to and diffusely coated the inner aspect of the pericardial sac. Poorly defined white homogeneous glossy tissue was present at the base of the heart. The heart was maximally contracted. The coelomic cavity contained ~4 mL of clear amber watery fluid (hydrocoelom). An ~1 × 1.3 cm diameter conglomerate of ~0.5 cm diameter, smooth, beige nodules was present next to both testes.
Laboratory results:
A 265-bp sequence of the internal transcribed spacer 1 (ITS1) region of the ribosomal RNA gene was 100% homologous with E. pogonae (GenBank accession KR998311).
Microscopic description:
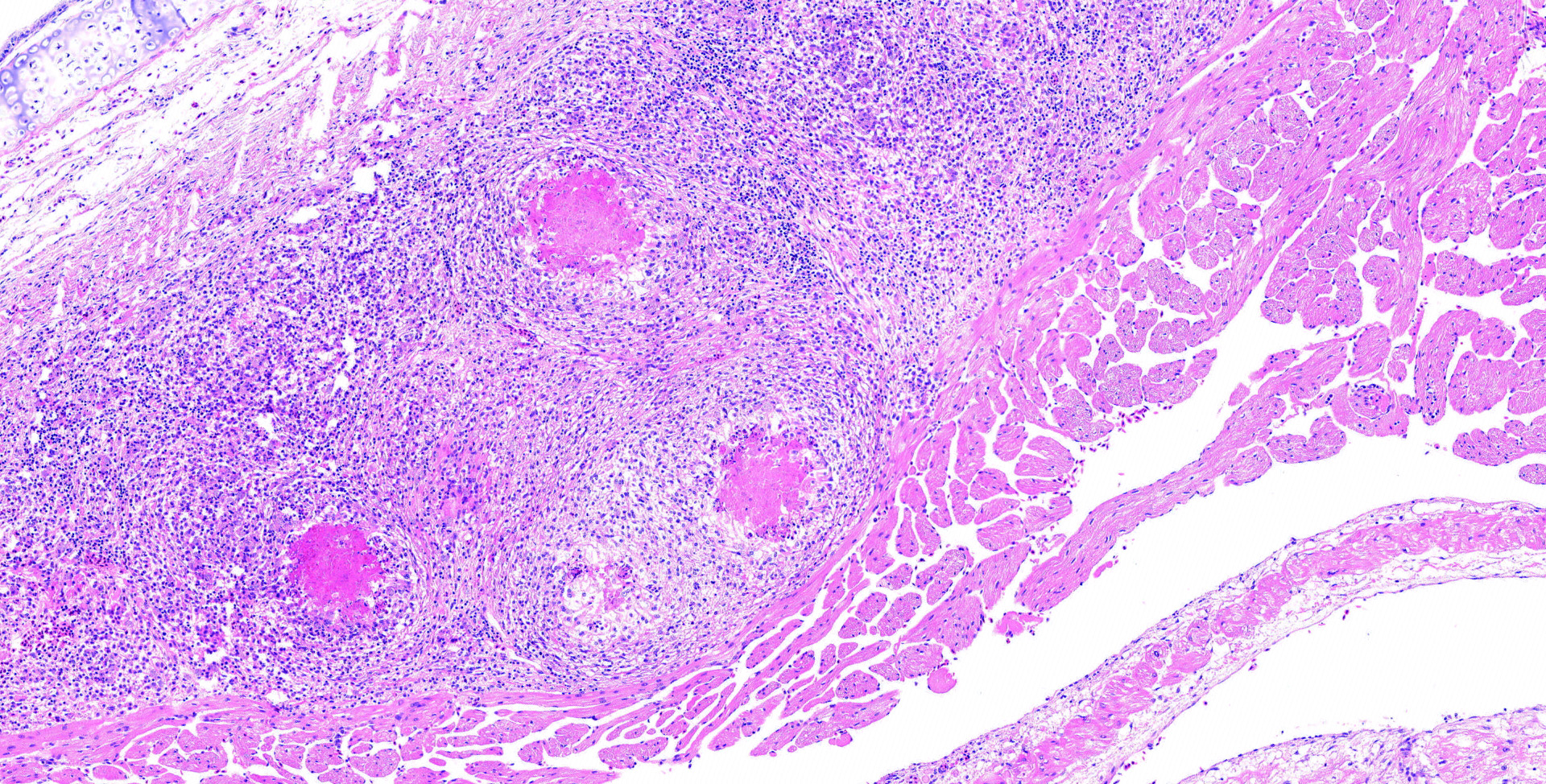
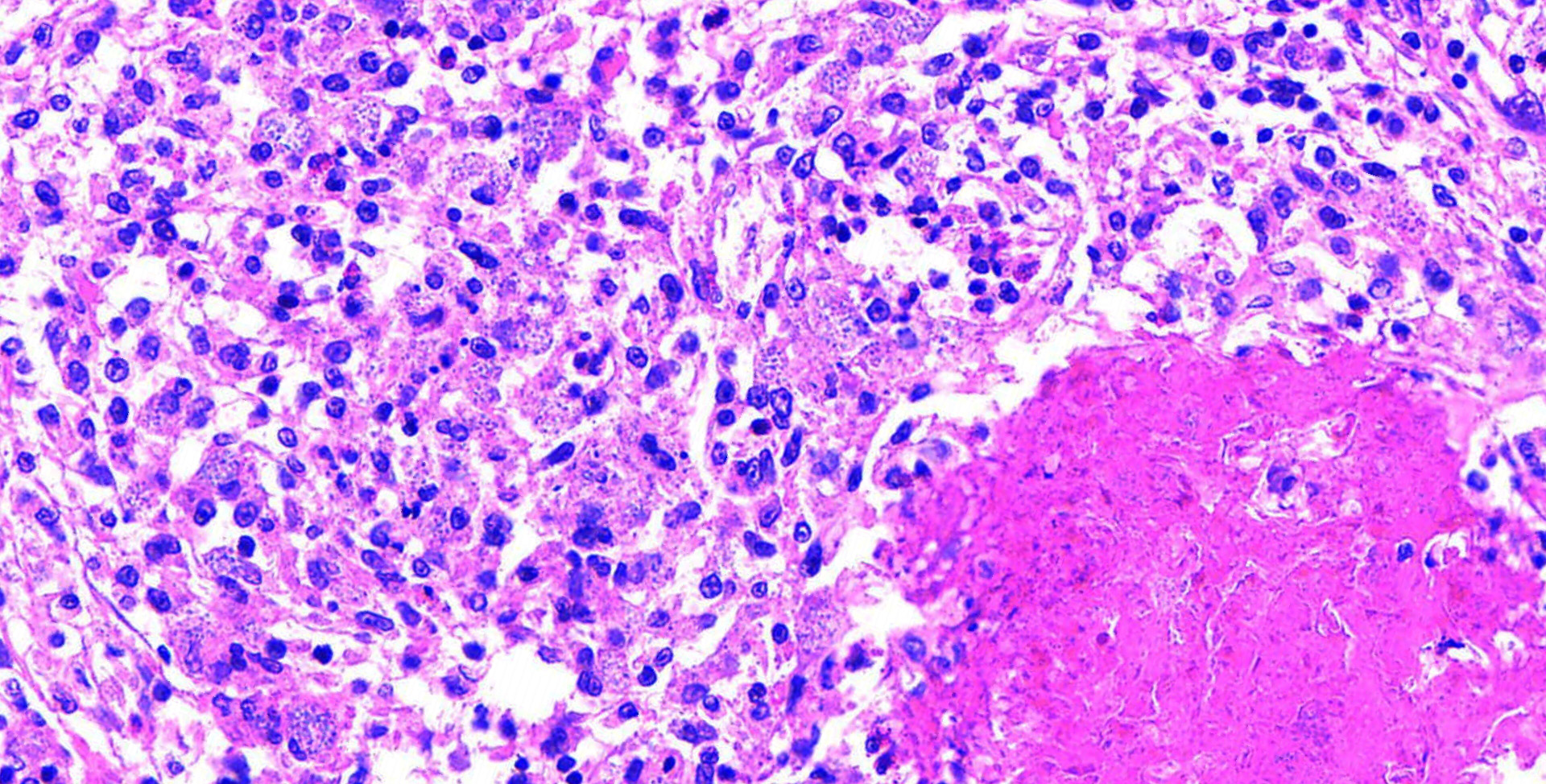
The slide contained a longitudinal section of the heart with the ventricle and one atrium. The epicardial surface of the atrium was expanded and covered by a densely cellular infiltrate largely composed of macrophages admixed with fewer heterophilic granulocytes. Multifocally within the atrial epicardial infiltrate, epithelioid macrophages were arranged around a core of eosinophilic debris. This debris contained numerous, weakly basophilic-staining microorganisms. These microorganisms were slightly elongated, up to 2 ?m long, and contained a vacuole. The cytoplasm of occasional macrophages was extended by similar microorganisms. The organisms were gram-positive and weakly PAS-positive but acid-fast?negative and negative with the GMS stain. Giemsa stain highlighted the wall and nucleus of the microorganisms. The epicardium of the ventricle and of one atrium (of the heart) were multifocally lined by a thick membrane of fibrous connective tissue. The connective tissue membrane covering the ventricle contained scattered mixed cellular inflammatory infiltrates (lymphocytes, plasma cells, macrophages and heterophilic granulocytes). The fibrous connective membrane covering the apex of the heart had subtle fibrin deposits on the outer surface.
On transmission electron microscopy, the microorganisms were identified as microsporidians based on the presence of exospore, endospore, vacuole, nucleus, and a polar filament. The organisms averaged 1.83 ?m long × 0.92 ?m wide. The polar filaments had 4?6 coils and averaged 61 nm in diameter.
Contributor's morphologic diagnosis:
Heart, epicarditis, granulomatous and heterophilic, widespread, chronic, marked with intrahistiocytic microorganisms (presumably microsporidia)
Contributor's comment:
The contributor writes in his most recent journal article on this subject:
"Microsporidia are atypical fungi that parasitize and cause disease in invertebrates, fish, amphibians, reptiles, and mammals.19 Microsporidia reproduce within the host cell, including macrophages, in which they are detectable histologically.3,18 The spores are oblong and have a clear vacuole. They measure up to 3 ?m long and ~1 ?m in diameter, although some species have larger spores.1 Mature spores are refractile, gram-positive, and have variable tinctorial properties when stained with silver stains, Giemsa stain, and when subjected to the PAS reaction.3 On electron microscopy, the spores are characterized by an exospore, endospore, and coiled polar tubular filament.9,11 The number of windings of this filament varies depending on species and developmental stage. Fatal and non-fatal microsporidian infections have been reported in central bearded dragons (syn. inland bearded dragon; Pogona vitticeps).6,10,12,14,15 In 2016, a microsporidian detected in a bearded dragon was identified as the new species Encephalitozoon pogonae.15"
The case presented was characterized by a cardiac manifestation that led to hemopericardium presumably as a result of hemorrhage from the granulation tissue and likely to an impaired diastolic expansion of the ventricle ("restrictive epicarditis").20 The hydrocoelom is evidence of heart failure, although the good nutritional state of the animal suggests that the disease did not negatively affect the animal for a prolonged time. Aneurysmal vascular disease is a common problem in bearded dragons; further investigation of the role of E. pogonae in vascular damage is indicated.16 The source of the microsporidian and the route of infection in both animals are uncertain. Bearded dragons are omnivores, and vegetables and insects usually comprise the majority of their captive diet. Insects harbor many known microsporidian organisms.19 The possibility of transmission of microsporidians from their insect prey to bearded dragons seems plausible but alternatively, fecal contamination of fruits and vegetables has been found to be a source of microsporidia.2 Vertical transmission of Encephalitozoon species has been documented in rabbits infected with E. cuniculi.5
Contributing Institution:
University of Minnesota Department of Veterinary Population Medicine/Minnesota Veterinary Diagnostic Laboratory - https://www.vetmed.umn.edu/departments/veterinary-population-medicine
JPC diagnosis:
Heart, epicardium: Epicarditis, granulomatous and granulocytic, diffuse, chronic, severe, with marked epicardial fibrosis, and numerous intrahistiocytic and extracellular microsporidial spores, bearded dragon, lizard.
JPC comment:
The contributor provides a short review of microsporidia and Encephalitozoon pogonae and is the author of the most recent journal article describing this species in the bearded dragon. This case provides context for a review of microsporidian anatomy and lifecycle.
Microsporidia are a diverse group of obligate intracellular, atypical fungi. The first microsporidian was described in the nineteenth century when "pepper disease" was afflicting silkworms in Europe. That particular agent was named Nosema bombycis in 1857, and at the time was considered a member of the schizomycete fungi. It was moved into the new classification 'Microsporidia' in 1882, which is now composed of more than 150 described genera and more than 1200 species.8
The microsporidian spore, as described by the contributor, has an exospore wall, an endospore wall, sporoplasm within the spore membrane, a single or two closely associated nuclei (diplokaryon), a polarplast, the polar filament (or polar tube), and the posterior vacuole. The polar tube is often the most distinctive feature on TEM, attached to the anchoring disk, and extends straight for a portion of the spore, then coils around the periphery of the spore. The number of coils, their arrangement, and angle of the coils are well conserved within species and are a useful diagnostic tool for identification.4,8
Oncotic pressure within the spore builds to critical levels, which is likely the initiator for germination. A complex sequence of events results in the polar filament being everted from the spore, varying in length from 50-500 ?m, at speeds exceeding 100 ?m/s. It is currently believed that the process of germination may result in an adjacent host cell being pierced by the polar filament, and then receiving sporoplasm (the infectious material of the spore) through the polar filament. The sporoplasm develops into meronts (merogony), followed by sporogony. Meronts give rise to sporoblasts, which develop into mature spores. Many species will be found within parasitophorous vacuoles in host cells.8
One way to stratify the microsporidian species is based upon their ability to induce xenoma formation. Xenomas are composed of a hypertrophied host cell within which reside microsporidians at one life stage (spores, meronts, etc), and has the appearance of a cyst-like structure in the host tissue. Some species known to induce xenoma formation include Glugea spp, Tetramicra spp, Loma spp, and others. Others such as Pleistophora spp, Nucleospora spp, Heterosporis spp, and others do not induce xenoma formation.13
Microsporidian infections are rarely described in canines (Encephalitozoon spp), lagomorphs (Encephalitozoon cuniculi and other species), falcons (Enterocytozoon bieneusi), psitticines, passerines, and crocodilians (Encephalitozoon hellem), amphibians (Pleistophora spp, Alloglugea bufonis, others), fish (Pleistophora spp, Glugea spp), and invertebrates (various species).17 Most species noted to affect humans are zoonotic and/or waterborne, and is a particular problem in immunocompromised individuals, such as HIV/AIDS patients. In many of these cases, Enterocytozoon bieneusi causes lethal infections, often starting with diarrhea. However, numerous other Microsporidian species have also been documented in humans as well.4
With additional research and documentation of cases, source and transmission route of Encephalitozoon pogonae may be ascertained, as well as whether vertical transmission is possible.
References:
- Cali A, Neafie RC, Takvorian PM. Microsporidiosis. In: Meyers WM, et al., eds. Topics on the Pathology of Protozoan and Invasive Arthropod Diseases. Washington, DC: Armed Forces Institute of Pathology, 2011:200?223.
- Clavo M, Carazo M, Arias ML, et al. Prevalencia de Cyclospora sp., Cryptosporidium sp., microsporidos y determinacion de coliformes fecales en frutas y vegetales frescos de consumo crudo en Costa Rica [Prevalence of Cyclospora sp., Cryptosporidium sp., microsporidia and fecal coliform determination in fresh fruit and vegetables consumed in Costa Rica]. Arch Latinoam Nutr 2004;54:428?432. Spanish
- Garcia LS. Laboratory identification of the microsporidia. J Clin Microbiol 2002;40:1892?1901.
- Han B, Weiss LM. Microsporidia: Obligate Intracellular Pathogens within the Fungal Kingdom. Microbiol Spectr. 2017;5(2): 10.1128/microbiolspec.FUNK-0018-2016. doi:10.1128/microbiolspec.FUNK-0018-2016.
- Hunt RD, King NW, Foster HL. Encephalitozoonosis: evidence for vertical transmission. J Infect Dis 1972;126:212?214.
- Jacobson ER, Green DE, Undeen AH, et al. Microsporidiosis in inland bearded dragons (Pogona vitticeps). J Zoo Wildl Med 1998;29:315?323.
7. Juan-Salles C, Garner MM, Dider ES, Serrato S, Acevedo LD, Ramos-Vera A, et al. Disseminated encephalitozoonosis in captive juvenile, cotton-top (Saguinus oedipus) and neonatal emperor tamarins (Saguinus imperator) in North America. Vet Pathol. 2006;43:438-446.
- Keeling PJ, Fast NM. Microsporidia: Biology and Evolution of Highly Reduced Intracellular Parasites. Annu. Rev. Microbiol. 2002;56:93-116.
- Lom J, Nilsen F. Fish microsporidian: fine structural diversity and phylogeny. Int J Parasitol 2003;33:107?127.
- Martel-Arquette A, Chen S, Hempstead J, et al. Microsporidial keratoconjunctivitis in a pet bearded dragon (Pogona vitticeps). J Exotic Pet Med 2017;26:257?262.
- Phelps NBD, Mor SK, Armien AG, et al. Description of the microsporidian parasite, Heterosporis sutherlandae n. sp., infecting fish in the Great Lakes region. PLoS One 2015;10:e0132027.
- Richter B, Csokai J, Graner I, et al. Encephalitozoonosis in two inland bearded dragons (Pogona vitticeps). J Comp Pathol 2013;148:278?282.
- Rodriguez-Tovar LE, Speare DJ, Markham F. Fish microsporidia: Immune response, immunomodulation and vaccination. Fish and Shellfish Immunology. 2011;30:999-1006.
- Shibasaki K, Tokiwa T, Sukegawa A, et al. First report of fatal disseminated microsporidiosis in two inland bearded dragons Pogona vitticeps in Japan. JMM Case Rep 2017;4:e005089.
- Sokolova YY, Sakaguchi K, Paulsen DB. Establishing a new species Encephalitizoon pogonae for the microsporidian parasite of bearded dragon Pogona vitticeps Ahl 1927 (Reptilia, Squamata, Agamidae). J Eukary Microbiol 2016;63:524?535.
- Sweet CR, Linnetz E, Golden E. What is your diagnosis? Pericardial effusion, cardiac mass, or cardiomegaly secondary to heart diseases. J Am Vet Med Assoc 2009;234:1259?1260.
- Terio KA, McAloose D, St. Leger, J. Pathology of Wildlife and Zoo Animals. San Diego, CA: Elsevier. 2018.
- Vávra J, Luke? J. Microsporidia and 'the art of living together'. Adv Parasitol 2013;82:253?319.
- Vergeneau-Grosset C, Larrat S. Microsporidiosis in vertebrate companion exotic animals. J Fungi (Basel) 2015;2:E3.
- Wünschmann A, Armién AG, Childress AL, et al. Intrapericardial Enzephalitozoon pogonae-associated arteritis with fatal hemopericardium min two juvenile bearded dragons. J Vet Diagn Invest 2019;31:467-470.