Signalment:
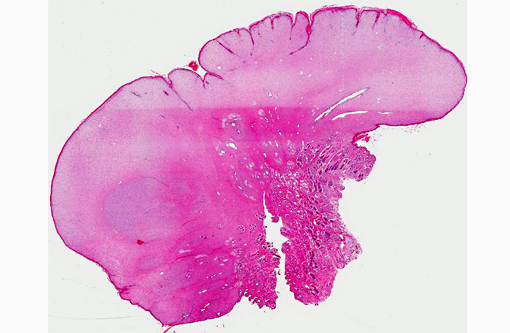
Gross Description:
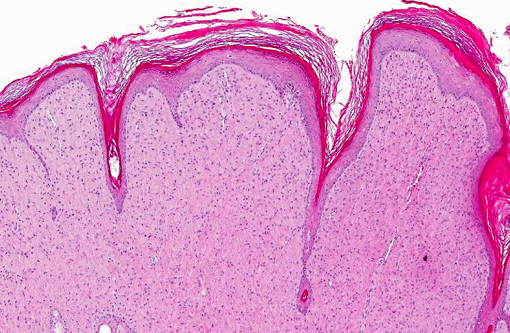
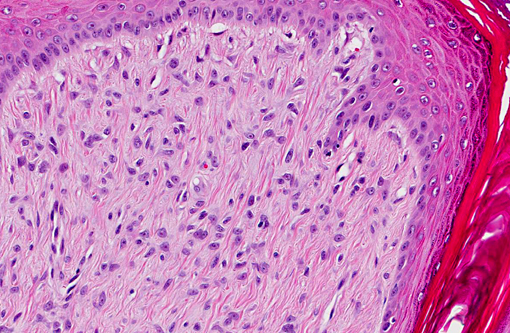
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Detection of papillomaviral DNA (PCR): positive
[Institute of Hygiene and Infectious Diseases of Animals, Justus Liebig University Giessen]
Detection of FeSarPV (PCR): positive
[Institute of Virology, Justus Liebig University Giessen]
Condition:
Contributor Comment:
Different papillomaviruses are suspected to be responsible for feline sarcoids. The feline sarcoid associated papillomavirus (FeSarPV) is similar to BPV-1, OvPV-1 and BPV-2. The genome of FeSarPV shows high homology with the genome of papillomaviruses of different ruminants.(22) Some of these papillomaviruses are classified as members of the genus Deltapapillomavirus. In contrast, the host specific papillomaviruses of cats, causing papillomatous plaques and real papillomas, are members of the genus Lambdapapillomavirus.(9) Infection of horses or cats by ruminant papillomaviruses is therefore regarded as cross-species papillomavirus infection.
For a recent classification of animal papillomaviruses, see Rector and van Ranst, 2013.(16)
Sarcoids are also described in lions. A possible mode of infection of large felids may be the consumption of bovine carcasses that had not been skinned.(15,17)
Viral papillomas caused by feline papillomavirus (FdPV-1) are rarely found in domestic cats.(22) These lesions are most likely associated with different forms of immunodeficiency in stray cats often in association with FIV infection.(2,11) Feline papillomatous plaques, often caused by FdPV-28, sometimes undergo malignant transformation to squamous cell carcinoma (SCC).(4,8,19) In one report, Human papillomavirus type 9 was identified using molecular biology.(13)
Predisposing factors of feline sarcoids are the behavior of rural cats. Contact with ruminants is occasionally mentioned. Numerous cases of sarcoids are reported from areas with dairy industry (e.g. New Zealand, Minnesota).(3,5,12,18) However, many interspecies contacts or modes of virus transmission are possible. A recent study demonstrated detection of feline sarcoid PV genome sequences within different bovine skin samples.(12)
Additional factors that favor the development of sarcoids in cats are in discussion, for example, trauma.(6) Interestingly, as in the presented case, FIV infection is reported in cases of feline sarcoids(21) and can play a role in the pathogenesis of the disease.
Tumors most often occur in young male cats. They are solitary or multiple skin nodules that measure up to 2 cm and they can be pedunculated or ulcerated. Predilection sites are the skin at the ears, lips, tail or paws. Their consistency is firm.(3,4,18)
Histomorphology of feline sarcoids is identical to equine sarcoids. Characteristically they show proliferating fibroblasts covered by hyperplastic epidermis.(18) Differentials are fibrosarcoma, histiocytic sarcoma, other spindle cell sarcomas, peripheral nerve sheath tumor and amelanotic melanoma.(3,4)
Feline sarcoids often show local recurrence after surgery. Often, relapses show a marked increase in growth rate.(4)
The presented case shares most of the characteristics described for that entity. Three months after surgery, the health status of the cat was good, and there were no signs of tumor recurrence.
JPC Diagnosis:
Conference Comment:
- Alpha (α): Oncogenic high risk mucosal types that cause benign mucosal/cutaneous lesions
- Beta (β): Cutaneous PVs that rarely cause lesions without immunosuppression
- Delta (δ): Cause benign fibropapillomas in ungulates; unique ability to infect multiple species (e.g. Bovine papillomavirus (BPV)-1 and -2 affects both cattle and horses)
- Epsilon (ε): BPV-5 and -8; cause both fibropapillomas and true papillomas
- Lambda (λ): Associated with skin lesions in the dog and cat; Felis domesticus PV1 (FdPV-1) and canine oral PV (COPV)
- Xipa (ζ): Bovine papillomaviruses (BPV)-3, -4, -6, -9 and -10; restricted to cattle and cause true, cutaneous squamous papillomas
Papillomaviruses are usually host specific, with a strong tropism for cutaneous and mucosal squamous epithelium, where they typically induce the formation of benign squamous papillomas or fibropapillomas. These tumors tend to spontaneously regress as a result of cell-mediated immunity; however, some PVs can undergo malignant transformation, leading to locally aggressive tumors such as equine sarcoids, or invasive squamous cell carcinomas (ISCC).(3,7,10)
In horses, infection with BPV-1 and -2 in areas subjected to trauma may induce the formation of a fibropapilloma, also known as equine sarcoid, which is grossly and histologically similar to the neoplasm described in this case.(3,10) The most important proteins expressed by equine sarcoids are BPV major transforming protein E5, BPV E6 protein and BPV E7 protein. BPV E5 binds the β-receptor of PDGF (PDGFβ-r) and activates kinases, such as cyclin A-cdk2, MAP, JNK, PI3 and c-Src, thus interfering with cell-cycle control and signal transduction cascades and ultimately promoting fibroblast growth as well as loss of contact inhibition. E5 also downregulates MHC1 expression, which allows infected cells to evade immunosurveillance. BPV E6 protein binds a calcium-binding protein (ERC-55/E6BP), a transcriptional co-activator (CBP/p300), a focal adhesion protein (paxillin), and the adaptor complex AP-1, which is important in control of cell proliferation and differentiation. Overall, these interactions lead to disruption of the cytoskeleton and cellcell/cellmatrix interactions, ultimately contributing to uncontrolled cellular proliferation. This is in contrast to Human papillomavirus (HPV) E6 protein, which acts by stimulating degradation of p53.(1,14) Furthermore, there is some evidence to suggest that co-expression of BPV E5 and E7 is necessary for neoplastic transformation in horses. In human mucosal alpha-PVs (e.g. HPV-16, -17), E7 binds and inactivates the tumor suppressor Rb, promoting cell cycling.(10) In addition to equine sarcoids induced by BPV-1 and -2, Equus caballus papillomavirus-2 (EcPV-2) has recently been identified in equine genital papillomas, in situ carcinomas (ISC) and ISCCs.(6)
In cats, FdPV-1 and -2 infection, in combination with solar-induced p53 mutation and papillomavirus-induced inhibition of keratinocyte apoptosis, may lead to uncontrolled cell proliferation, progressing to Bowenoid in situ carcinoma (BISC) and, less commonly, SCC. Additionally, as noted by the contributor, papillomavirus DNA has been localized to proliferating fibroblasts suggesting an association between feline fibropapillomas (sarcoids) and PV.(10) As in cats, cutaneous PV infections in dogs can generate viral plaques that may subsequently progress to ISC or ISCC; pugs, miniature schnauzers and immunosuppressed dogs are predisposed. Although Canine oral papillomavirus (COPV) infection is not associated with cutaneous neoplasia, vaccination with a live COPV vaccine may result in cutaneous ISCC.(10)
There are currently ten different papillomaviruses described in cattle.(14,20) BPV-1 and BPV-2 cause fibropapillomas, while BPVs-3, -4, -6, -9 and -10 are epitheliotropic and induce true cutaneous squamous papillomas. BPV-5 and BPV-8 appear to have a dual pathology, causing both fibropapillomas and cutaneous squamous papillomas. Bracken fern (Pteridium aquilinum) contains immunosuppressants and a number of mutagens; in cattle that have ingested bracken fern, BPV-4-induced alimentary papillomas may progress to SCC, while transforming protein E5 associated with BPV-2 or -4 may synergize with ptaquiloside to produce bladder cancer.(14) See table 1 for a summary of select papillomaviruses and their affects on various species.
Table 1: Select papillomaviruses in domestic animal species.(3,6,7,10,14,20)
| Species | Skin lesion | Papillomavirus | Type |
| Cat | Feline viral plaque progressing to BISC +/- ISCC | FdPV-1, -2 | λ(1) |
| Cat | SCC | FdPV-2 | novel |
| Cat | Feline sarcoid (feline cutaneous fibropapilloma) | FeSarPV BPV-1,-2 | novel δ |
| Dog | Canine pigmented viral plaque progressing to ISC and SCC | CfPV-3, -4 | novel |
| Dog | Endophytic papilloma and SCC in immunosuppressed dogs | CfPV-2 | novel |
| Dog | Oral papilloma and vaccine-induced cutaneous SCC | COPV | λ |
| Horse | Equine sarcoid (equine cutaneous fibropapilloma) | BPV-1, -2 | δ |
| Horse | Equine penile papillomas, ISC & SCC | EcPV2 | |
| Ox | Cutaneous fibropapilloma | BPV-1,-2 | δ |
| Ox | Both fibropapillomas and squamous papilloma | BPV-5,-8 | Epsilon(ε) |
| Ox | Cutaneous squamous papilloma | BPV-3, -6, -9, -10 | Xi (ξ) |
| Ox | Squamous papilloma of alimentary tract & urinary bladder | BPV-4 | Xi (ξ) |
| Ox | Co-carcinogen with bracken fern (ptaquiloside) to induce urinary bladder neoplasms | BPV-2 | δ |
| Bull (young) | Papilloma/fibropapilloma of glans penis | BPV-1 | δ |
| Ox | Squamous papilloma | BPV-7 | novel |
| Cotton-tail rabbit | Cutaneous SCC | CRPV | Kappa (κ) |
| Western barred bandicoot | Cutaneous papillomatosis & SCC (digits, lips) | BPCV-1 | novel |
| Egyptian fruit bat | Basosquamous carcinoma | RaPV-1 | novel |
| Natal multimammate mouse | Keratoacanthoma and SCC | MnPV | Iota (ι) |
| European harvest mouse | Sebaceous carcinoma | MmPV | Pi (Ï) |
References:
2. Egberink HF, Berrocal A, Bax HA, van den Ingh TS, Walter JH, Horzinek MC. Papillomavirus associated skin lesions in a cat seropositive for feline immunodeficiency virus. Vet Microbiol. 1992;31:117-125.
3. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol 1. Philadelphia, PA: Elsevier Limited; 2007:553-781.
4. Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin diseases of the dog and cat: clinical and histopathologic diagnosis. 2nd ed. Oxford, UK: Blackwell Science Ltd; 2005:567-589, 730-731.
5. Hanna PE, Dunn D. Cutaneous fibropapilloma in a cat (feline sarcoid). Can Vet J. 2003;44:601-602.
6. Lange CE, Tobler K, Lehner A, et al. EcPV2 DNA in equine papillomas and in situ and invasive squamous cell carcinomas supports papillomavirus etiology. Vet Pathol. 2012;50:686-692.
7. MacLachlan NJ, Dubovi EJ. Fenners Veterinary Virology. 4th ed. London, UK: Academic Press; 2011:213-221.
8. Munday JS, Peters-Kennedy J. Consistent detection of Felis domesticus papillomavirus 2 DNA sequences within feline viral plaques. J Vet Diagn Invest. 2010;22:946-949.
9. Munday JS, Knight CG, Howe L. The same papillomavirus is present in feline sarcoids from North America and New Zealand but not in any non-sarcoid feline samples. Vet Diagn Invest. 2010;22:97-100.
10. Munday JS, Kiupel M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet Pathol. 2010;47:254-264.
11. Munday JS, Witham AI. Frequent detection of papillomavirus DNA in clinically normal skin of cats infected and noninfected with feline immunodeficiency virus. Vet Dermatol. 2010;21:307-310.
12. Munday JS, Knight CG. Amplification of feline sarcoid-associated papillomavirus DNA sequences from bovine skin. Vet Dermatol. 2010;21:341-344.
13. Munday JS, Hanlon EM, Howe L, Squires RA, French AF. Feline cutaneous viral papilloma associated with human papillomavirus type 9. Vet Pathol. 2007;44:924-927.
14. Nasir L, Saveria Campo M. Bovine papillomaviruses: their role in the aetiology of cutaneous tumours of bovids and equids. Vet Dermatol. 2008;19(5):243-254.
15. Orbell GM, Young S, Munday JS. Cutaneous sarcoids in captive African lions associated with feline sarcoid-associated papillomavirus infection. Vet Pathol. 2011;48:1176-1179.
16. Rector A, Van Ranst M. Animal papillomaviruses. Virology. 2013 (in press). 17. Schulman FY, Krafft AE, Janczewski T, Mikaelian I, Irwin J, Hassinger K. Cutaneous fibropapilloma in a mountain lion (Felis concolor). J Zoo Wildl Med. 2003;34:179-183.
18. Schulman FY, Krafft AE, Janczewski T. Feline cutaneous fibropapillomas: clinicopathologic findings and association with papillomavirus infection. Vet Pathol. 2001;38:291-296.
19. Schwittlick U, Bock P, Lapp S, Henneicke K, Wohlsein P. [Feline papillomavirus infection in a cat with Bowen-like disease and cutaneous squamous cell carcinoma]. Schweiz Arch Tierheilkd. 2011;153:573-577.
20. Silvestre O, Borzacchiello G, Nava D, et. al. Bovine papillomavirus type 1 DNA and E5 oncoprotein expression in water buffalo fibropapillomas. Vet Pathol. 2009;46:636-641.
21. Sundberg JP, Van Ranst M, Montali R, Homer BL, Miller WH, Rowland PH, et al. Feline papillomas and papillomaviruses. Vet Pathol. 2000;37:1-10.
22. Teifke JP, Kidney BA, L+�-�hr CV, Yager JA. Detection of papillomavirus-DNA in mesenchymal tumour cells and not in the hyperplastic epithelium of feline sarcoids. Vet Dermatol. 2003;14:47-56.