Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 19
April 4th, 2018
CASE III: W1161-16 (JPC 4102120).
Signalment: 15-year-old, male, neutered, Saluki (Canis familiaris), canine.
History: The dog had been previously diagnosed with hyperadrenocorticism based on low-dose dexamethasone suppression testing, and treatment with trilostane was initiated. The dog was transported a long distance by plane and subsequently became inappetent. The animal was found collapsed in a kennel and taken to the University of Melbourne veterinary referral service. At admission, the animal was recumbent and obtunded, with normal cranial nerve assessment. The dog was markedly tachycardic (180bpm) with a grade 1/6 left sided systolic heart murmur; an ECG revealed intermittent supraventricular tachycardia and occasional 2nd degree heart block. Body temperature was 39.8 ºC. Biochemistry, hematology and urinalysis results are presented below. The owner elected for the dog’s euthanasia following consultation.
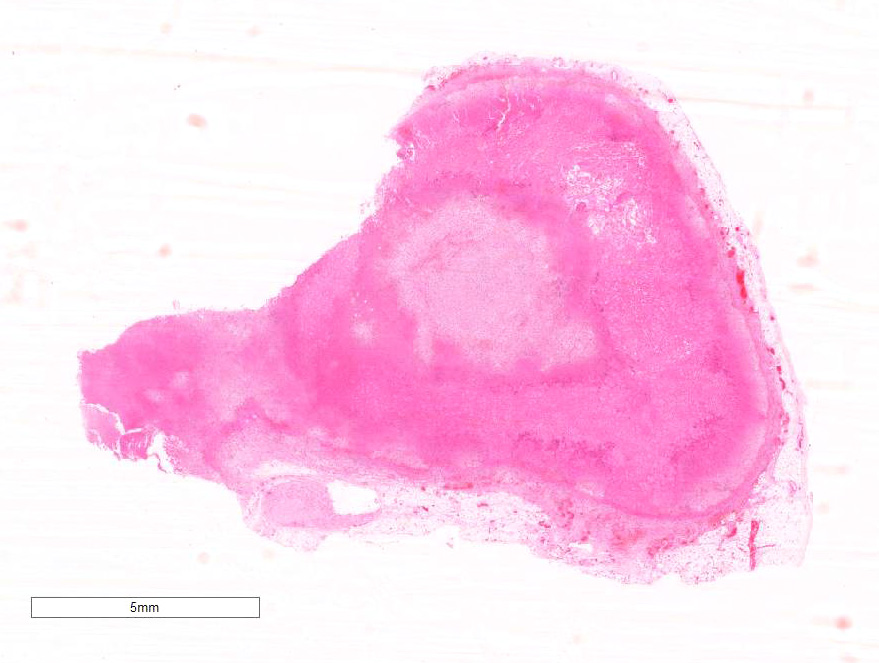
Gross Pathology: The liver was enlarged with rounded lobe margins and mottled red-brown coloration. The parenchyma was faintly nodular in structure and very friable. Within both adrenals there were multiple poorly-defined, variably sized, tan to beige-colored soft tissue nodules, and multifocally within the adrenal parenchyma there were regions of dark red to black discoloration. The renal surface was irregularly indented, and underlying the indented areas there were wedge-shaped foci of parenchymal pallor extending through the cortex into the medulla.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): see below
|
Haematology |
Value |
Canine Ref Values |
|
PCV % |
66 |
37-55 |
|
RBC (x1012/L) |
10.13 |
5.65-8.87 |
|
Hb g/dL |
24.9 |
8-12 |
|
MCV (fL) |
64.9 |
61.6-73.5 |
|
MCH (pg) |
23.2 |
21.2-25.9 |
|
MCHC (g/dL) |
35.9 |
32.0-37.9 |
|
Neut (x109/L) |
8.53 |
2.95-11.64 |
|
Lymph (x109/L) |
1.29 |
1.05-5.10 |
|
Mono (x109/L) |
0.42 |
0.16-1.12 |
|
Eosino (x109/L) |
0.6 |
0.06-1.23 |
|
Baso (x109/L) |
0.01 |
0.0-0.1 |
|
Platelet (K/µL) |
325 |
148-484 |
|
PT |
16 |
14-19 |
|
aPTT |
88 |
75-105 |
|
|
|
|
|
Biochemistry |
|
|
|
Urea (mmol/L) |
26.9 |
2.5-9.6 |
|
Crea (µmol/L) |
460 |
44-159 |
|
Phos (mmol/L) |
2.85 |
0.81-2.2 |
|
TP (g/L) |
61 |
52-82 |
|
Albumin (g/L) |
29 |
22-39 |
|
Globulin (g/L) |
32 |
25-45 |
|
ALT (U/L) |
239 |
10-125 |
|
ALKP (U/L) |
88 |
23-212 |
|
GGT (U/L) |
0 |
0-11 |
|
T Bil (µmol/L) |
6 |
0-15 |
|
Chol (mmol/L) |
5.48 |
2.84-8.26 |
|
Amylase (U/L) |
1485 |
500-1500 |
|
Lipase (U/L) |
422 |
200-1800 |
|
Glu (mmol/L) |
4.6 |
3.3-6.1 |
|
Lactate (mmol/L) |
1.3 |
<2.0 |
|
K+ (mmol/L) |
5.9 |
3.6 – 5.8 |
|
Na+ (mmol/L) |
140 |
145 – 158 |
|
Ca2+ (mmol/L) |
2.42 |
1.98-3.00 |
|
Cl- (mmol/L) |
115 |
105 – 122 |
|
|
|
|
|
|
|
|
|
Blood gases |
|
|
|
FiO2 % |
.21 |
|
|
A or V |
V |
V |
|
pH |
7.314 |
7.405 |
|
pCO2 mmHg |
25.2 |
36.6 |
|
pO2 mmHg |
34.5 |
52 |
|
HCO3 mEq/L |
12.4 |
22 |
|
Anion Gap mmol/L |
17.7 |
<24 |
Urinalysis: USG 1.018, pH 6, trace protein. Rare casts and no bacteria noted on urine sediment exam.
LDDST: Cortisol 228 nmol/L at 0h (28-150 nmol/L), 128 nmol/L at 4h (<30 nmol/L), and 220 nmol/L at 8h (<30 nmol/L). (References in parenthesis)
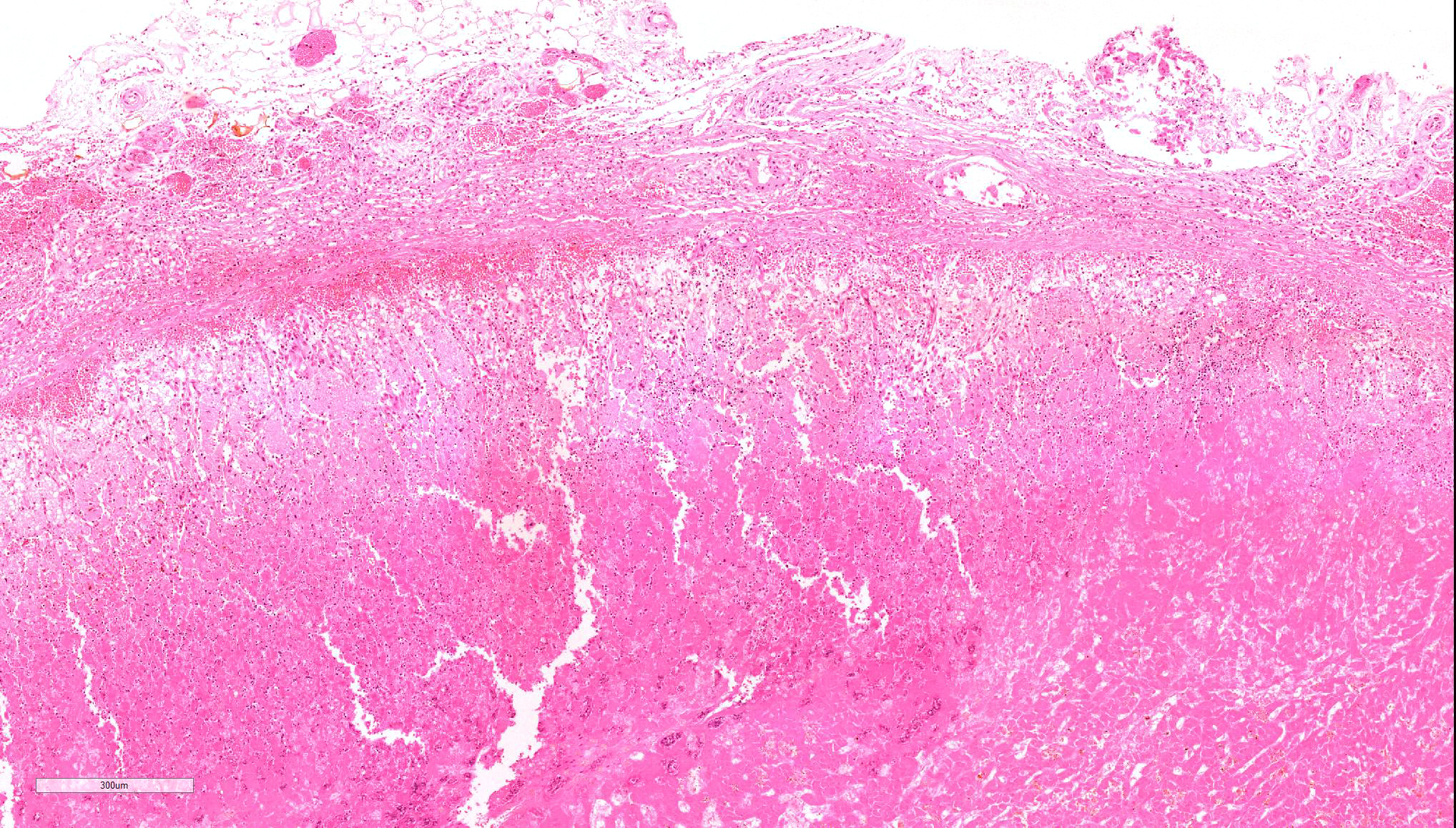
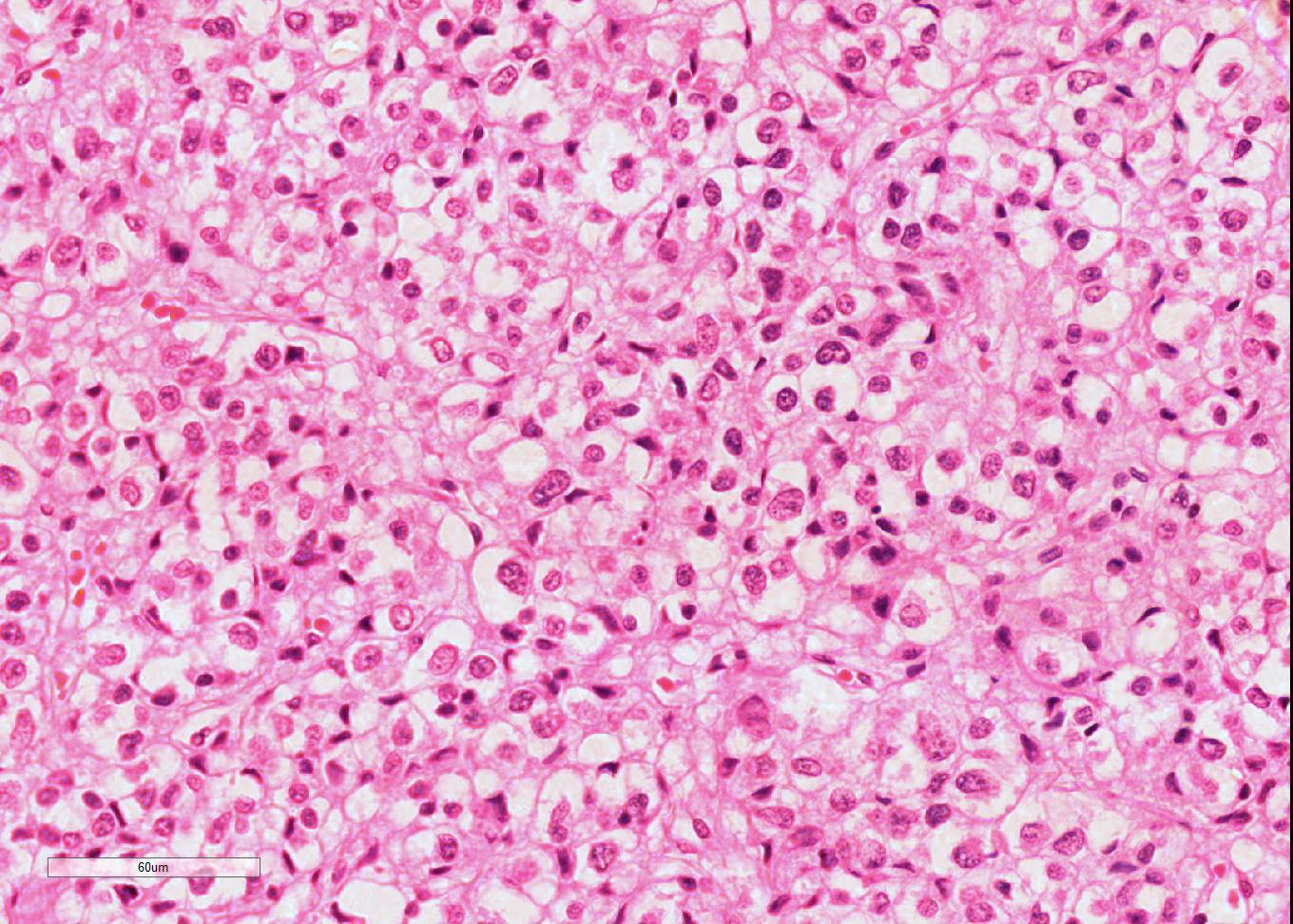
Microscopic Description: Diffusely throughout the adrenal cortex, there is loss of cellular detail, eosinophilic parenchymal homogeneity, and effacement of normal tissue architecture (necrosis). Multifocally within the affected areas there are deposits of lacy basophilic material (chromatin strands) and cellular debris which sometimes displays fine acicular clefting (cholesterol deposits), and there are also large regions that are suffused by extravasated erythrocytes (hemorrhage). Cortical cells in residual viable areas are poorly cohesive and are often swollen with cytoplasmic clearing (ballooning degeneration). There is relative sparing of the adrenal medullary structure, but the vasculature is moderately engorged and chromaffin cells display cytoplasmic eosinophilia (not present in all sections). Surrounding the areas of necrosis, there is a marked infiltrate of neutrophils (predominantly degenerate) and macrophages, which extends beyond the capsule, and there is also a poorly organized population of fibroblasts within early granulation tissue. The connective tissue surrounding the adrenal is expanded and rarefied (edematous) with engorged vessels, multifocal hemorrhage and deposits of lacy eosinophilic material (fibrin).
Contributor’s Morphologic Diagnosis:
Adrenal: Adrenocortical necrosis, severe, bilaterally diffuse, subacute
Contributor’s Comment: Adrenal necrosis is infrequently identified in domestic species, with foci of necrosis identified in 1.8% of cats (n=159) and 3.0% of dogs (n=101) in a recent survey.5 The necrosis in this case was attributed to an idiosyncratic response to trilostane therapy, resulting in secondary adrenocortical insufficiency and Addisonian crisis. Trilostane is commonly used for medical treatment of canine hyperadrenocorticism, and has superseded op’DDD as the drug of choice for control of this condition. It is a steroid analogue that reversibly inhibits adrenal steroidogenesis through blockade of 3β-hydroxysteroid dehydrogenase, thereby preventing the conversion of 3β-hydroxysteroids (pregenolone, 17-hydroxypregnenolone, and dehydroepiandrosterone) to 3-ketosteroids (progesterone, 17-hydroxyprogesterone, and androstenedione).10 Administration inhibits both mineralocorticoid and glucocorticoid synthesis, and sex hormone synthesis may also be impaired, although typically to a lesser degree. The metabolism of trilostane has not been examined in detail in dogs, but in rats the drug is partially converted to ketotrilostane by the liver before being excreted fecally, while urinary excretion predominates in monkeys.9
There are multiple published reports of adrenal necrosis in dogs associated with trilostane administration.2,11,12 Reutsch et. al. identified adrenal necrosis in five of seven dogs treated with trilostane for hyperadrenocorticism.12 This side-effect is not readily explained by the known effects of trilostane within the adrenal gland, and it has been proposed that the adrenal necrosis observed during trilostane therapy may reflect excessive secretion of adrenocorticotropic hormone (ACTH), rather than direct effects of the drug or its metabolites. This hypothesis is supported by trials demonstrating the development of adrenocortical hemorrhage and vacuolization in rats treated with ACTH, whereas treatment with trilostane alone produced no histological lesions.1 Moreover, adrenal hemorrhage has been observed clinically in humans receiving exogenous ACTH therapy.7 The exact role of trilostane in the pathogenesis of adrenocortical degeneration remains uncertain; however, it is possible that trilostane may promote hypersecretion of ACTH, or alternatively sensitizes adrenocortical cells to the toxic effects of ACTH. In the present case, we speculate that the stress of the flight prior to presentation may have precipitated excessive ACTH secretion and development of subsequent adrenal necrosis.
There are a large range of other chemicals capable of causing adrenal toxicity, as indicated in the table 1. The adrenal cortex is relatively susceptible to toxic effects due to its well-developed and highly permeable blood supply, its robust lipid metabolic pathways with strong uptake of lipophilic substances, and its abundance of cytochrome P450 enzymes for biotransformation. Toxins may be selective in the region of the adrenal affected, even within the different cortical zones, and thus identifying the affected regions may aid in determining the causative agent. Compounds such as aniline and sulfated mucopolysaccharides predominantly target the zona glomerulosa, whereas the effects of toxins such carbon tetrachloride, acrylonitrile, clotrimazole and op’-DDD are largely confined to the zonas fasciculata and reticularis.13 Toxicity affecting the adrenal medulla is relatively uncommon but has been reported with compounds such as reserpine, thiouracil and xylitol; cellular proliferative changes appear to be the most common manifestation of medullary toxicity.13
Non-toxic causes of adrenal degeneration may also occur. Hemorrhage and necrosis predominantly affecting the adrenal cortex has been observed in association with septic infections in humans, in particular those caused by Neisseria meningitidis, Staphylococcus aureus and streptococci. The pathogenesis of this condition - known as Waterhouse-Friederichsen Syndrome – is poorly understood, but it has been proposed that adrenaline release may induce both platelet aggregation and marked adrenal vasoconstriction, predisposing to venous thrombosis and infarction within the gland, particularly in association with concurrent disseminated intravascular coagulation.9 Similar changes may be observed in septicemic horses5 and calves15, as well as in young lambs dying from exposure. Although possibly not completely analogous, adrenocortical hemorrhage may also be present in horses that die of during marked exertion. Adrenal hemorrhage has been induced experimentally in rabbits through intravascular endotoxin administration8, but it is interesting to note that adrenocortical hemorrhage does not develop in hypophysectomized animals treated with endotoxin, suggesting that the pathogenesis of Waterhouse-Friederichsen syndrome requires pituitary signaling. Thus, aberrant ACTH secretion may be a common factor in the pathogenesis of both trilostane toxicity and septic adrenal hemorrhage.
Table 1. Adrenal toxins (from Colby4)
|
Acrylonitrile ACTH Aflatoxin Aminoglutethimide Aniline Bromocripitine Carbon tetrachloride Chenodeoxycholic acid Chloroform Chlorphentermine Clotrimazole Cyproterone Cysteamine hydrochloride op'-DDD Danazol
|
Dilantin Dimethylbenzanthracenes Estrogens Ethanol Etomidate Fluphenazine Hexadimethrine bromide Iprindole Ketoconazole Mefloquine Methanol Nitrogen oxides Parathion PBBs, PCBs Polyanthosulfonate+ aminocaprionic acid |
Polyglutamic acids Ponceau SX Pyrazole Spironolactone Sulfated mucopolysaccharides Suramin Tamoxifen Tetrachlorvinphos Testosterone Thioacetamide Thioguanine Toxaphene Triparanol Urethane Zimelidine |
JPC Diagnosis: Adrenal gland: Necrosis, cortical and medullary, diffuse, severe with hemorrhage, Saluki (Canis familiaris), canine.
Conference Comment: The word “adrenal” comes from the Latin for near (ad-) and kidney (renes) thus named for its relative location to the kidneys. An Italian anatomist, Bartolomeo Eustachi, is the individual credited with their discovery in 1563. However, his works were not received publicly until years later because they were secluded in the papal library. Eustachius (as he was known) along with Vesalius are considered the fathers of human anatomy. Due to religious restrictions on anatomists through the Renaissance, his anatomy book became a bestseller more than a century after his death.3 His works are broken down into 17 “plates” in which he describes and illustrations the kidneys, ear, heart (including the vena azygous and vena cava, both named by him), thoracic and abdominal viscera, brain, spinal cord, and detailed descriptions of peripheral nerves.14 In order to more clearly see the intimate structure and detail of these organs, Eustachius created magnifying glasses (early microscopes) and used different fluids to break down tissues. As you may expect, he also had extended knowledge of the inner ear, naming the Eustachian tube and diagraming the malleus, stapdius, and cochlea.16
The conference moderator discussed the submitted laboratory work, initially keying in on the azotemia (in which creatinine and urea are markedly increased), the elevated PCV (dehydration), and the urine specific gravity indicating dilute urine (1.018) with casts. Due to those findings, he favored renal rather than pre-renal azotemia. Conversely, he also pointed out that there was no stress leukogram, which is abnormal for a sick dog and favors Addison’s disease. After discussion with the contributor and reviewing the gross description of the kidney, the moderator concluded that this patient must have had concurrent renal disease which is what caused the clinical pathological findings. Additionally, he favors infarction of the gland rather than the toxic effects of trilostane administration. Conference attendees noted that trilostane administration is contraindicated in renal failure even though it is metabolized by the liver and excreted fecally.
Contributing Institution:
Veterinary Anatomic Pathology
Faculty of Veterinary and Agricultural Sciences
The University of Melbourne
Victoria, Australia
References:
- Burkhardt WA, Guscetti F, Boretti FS, Ivos Todesco A, Aldajarov N, Lutz TA, et al. Adrenocorticotropic hormone, but not trilostane, causes severe adrenal hemorrhage, vacuolization, and apoptosis in rats. Domest Anim Endocrinol. 2011; 40(3): 155-164.
- Chapman PS, Kelly DF, Archer J, Brockman DJ, Neiger R. Adrenal necrosis in a dog receiving trilostane for the treatment of hyperadrenocorticism. J Small Anim Pract. 2004; 45(6): 307-310.
- Choulant L. History and Bibliography of Anatomic Illustration. Translated and annotated by Mortimer Frank. New York, NY: Hafner; 1962:200-204.
- Colby HD. Adrenal-gland toxicity - chemically-induced dysfunction. Journal of the American College of Toxicology. 1988; 7(1): 45-69.
- Hart KA, Barton MH. Adrenocortical insufficiency in horses and foals. Vet Clin North Am Equine Pract. 2011; 27(1): 19-34.
- Herbach N, Wiele K, Konietschke U, Hernmanns W. Pathologic alterations of canine and feline adrenal glands. Open Journal of Pathology. 2016; 6: 140-153.
- Kornbluth AA, Salomon P, Sachar DB, Subramani K, Kramer A, Gray CE, et al. ACTH-induced adrenal hemorrhage: a complication of therapy masquerading as an acute abdomen. J Clin Gastroenterol. 1990; 12(4): 371-377.
- Levin J, Cluff LE. Endotoxemia and adrenal hemorrhage. A mechanism for the Waterhouse-Friderichsen syndrome. J Exp Med. 1965; 121: 247-260.
- Piccioli A, Chini G, Mannelli M, Serio M. Bilateral massive adrenal hemorrhage due to sepsis: report of two cases. J Endocrinol Invest. 1994; 17(10): 821-824.
- Ramsey IK. Trilostane in dogs. Vet Clin North Am Small Anim Pract. 2010; 40(2): 269-283.
- Ramsey IK, Richardson J, Lenard Z, Tebb AJ, Irwin PJ. Persistent isolated hypocortisolism following brief treatment with trilostane. Aust Vet J. 2008; 86(12): 491-495.
- Reusch CE, Sieber-Ruckstuhl N, Wenger M, Lutz H, Perren A, Pospischil A. Histological evaluation of the adrenal glands of seven dogs with hyperadrenocorticism treated with trilostane. Vet Rec. 2007; 160(7): 219-224.
- Ribelin WE. The effects of drugs and chemicals upon the structure of the adrenal gland. Fundam Appl Toxicol. 1984; 4(1): 105-119.
- Roberts KB. Eustachius and his anatomical plates. Newsletter of the Canadian Society for the History of Medicine. 1979; Apr: 9-13.
- Rosol TJ, Grone A. Endocrine glands. In: Maxie G, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. Vol. 3. 6th St. Louis, MO: Elsevier Health Sciences; 2015: 269-357.
- Schmidt JE. Medical Discoveries: Who and When. Thomas Publishing; 1959: 9-10.