Signalment:
Gross Description:
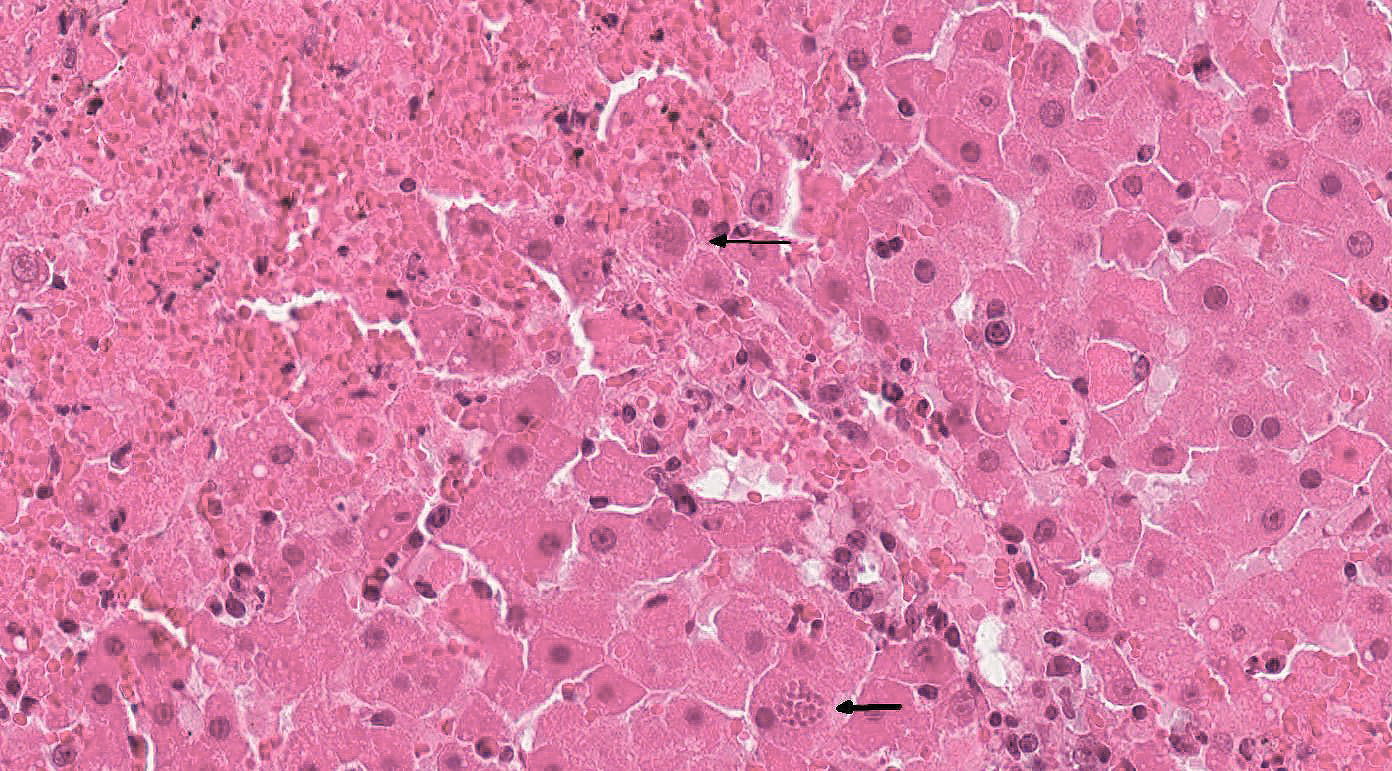
Histopathologic Description:
Within necrotic foci and within the cytoplasm of adjacent hepatocytes are numerous protozoal cysts measuring 15 x 20 µm with a thin capsule and containing numerous 1-2 µm elongated zoites. Free within the necrotic areas are 1-2 µm elongated tachyzoites.
Portal areas are infiltrated by moderate numbers of lymphocytes, plasma cells, and eosinophils, often aggregated around bile ducts. There is a moderate increase of the number of bile ducts (hyperplasia). Within the bile duct epithelial cells, moderate numbers of large, round to oval coccidial oocysts, about 20-40 µm in diameter, with a 1-2 µm thick eosinophilic wall, lightly basophilic granular cytoplasm, and one nucleus are visible.
Morphologic Diagnosis:
2. Liver: Cholangitis, lymphoplasmacytic and eosinophilic, multifocal, moderate, subacute, with bile duct hyperplasia and intraepithelial coccidial oocysts: etiology consistent with Cyclospora talpae
Lab Results:
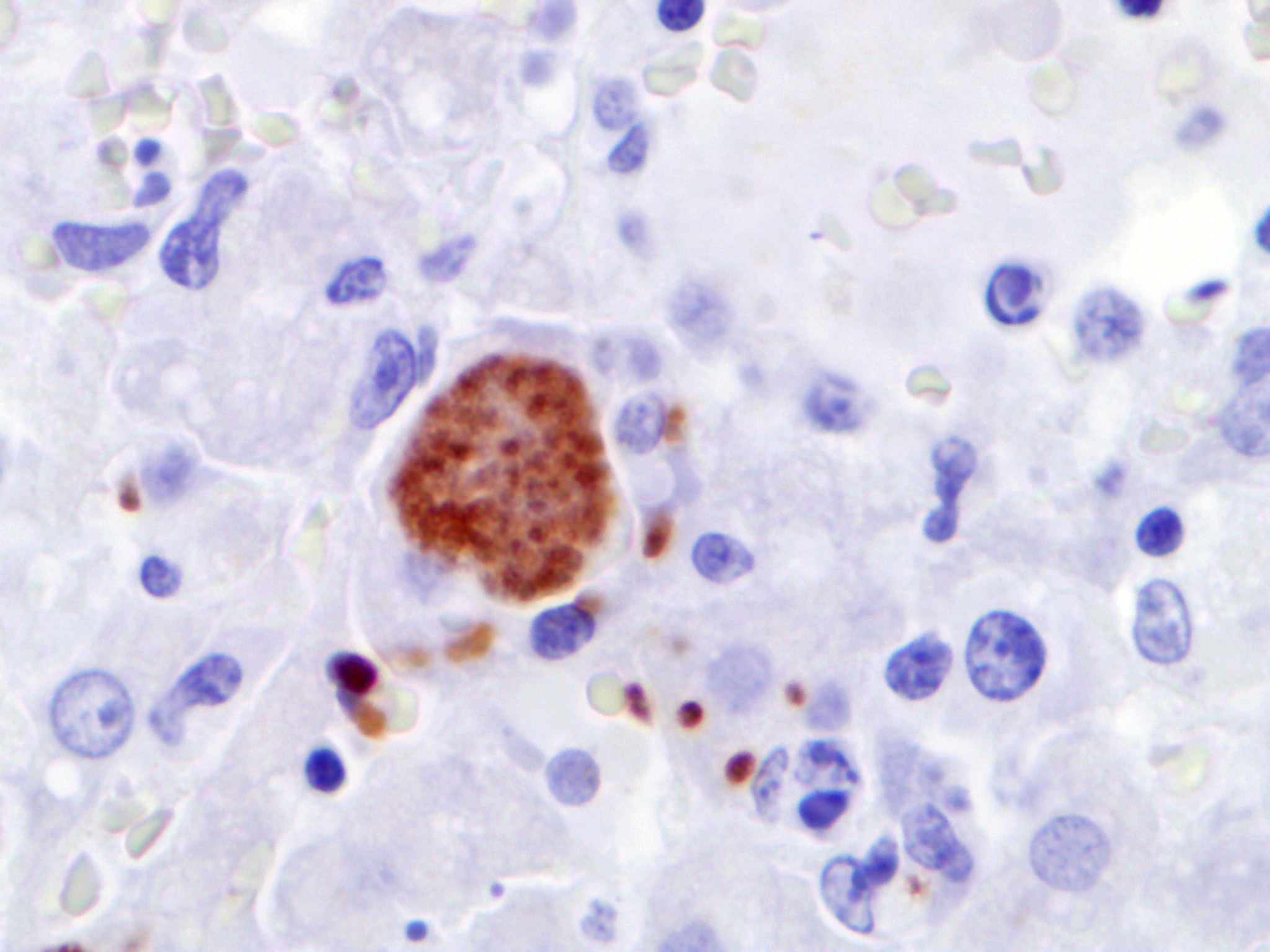
Immunohistochemically, antigen of Toxo-plasma gondii is detectable within liver and spleen using a polyclonal antiserum and the PAP method. Immunohistochemistry to detect antigen of Neospora caninum is negative in all tissues.
An attempt to amplify specific genomic sequences of Toxoplasma gondii as well as Neospora caninum using formalin fixed, paraffin embedded material of liver is unsuccessful (may be due to the long fixation time of the tissue samples in unbuffered formalin).
Condition:
Contributor Comment:
Only a few parasites have been described in moles and other insectivores.5,11 However, it is known that Cyclospora talpae can be found in the epithelium of bile ducts in the liver and share similarities with Eimeria stiedae in rabbits. Toxoplasmosis has also been described in moles. In 1995, a similar case of combined toxoplasmosis and cyclosporiasis was documented in Bavaria.9
Due to their behavior, moles are a fossorial species that have extensive contact with soil and they can serve as intermediate hosts for Toxoplasma gondii. Moles also eat earthworms, which are identified as paratenic or transport hosts for these protozoans.2,13 Seroprevalence for Toxo-plasma gondii is about 40 % in wild moles in France.1
Toxoplasma gondii is a zoonotic protozoan which infects most mammalian and avian species. It is one of the most ubiquitous parasites and almost all homoeothermic species can be infected experimentally.6 In domestic animals, overt disease is rare with the exception of abortion in sheep and goats. While felids are the definitive hosts (cats) and shed infective oocysts, the intermediate hosts (including cats) harbor parasitic stages in different tissue.8,14 Predisposing factors for systemic toxoplasmosis are in-sufficiencies of the immune system (e.g. low levels of gamma-interferon) or concomitant infections. Recent observations regarding the pathogenesis of Toxoplasma gondii show that different TLRs are involved in the recognition of the parasite and there are also obvious differences between man and animal models in the effector mechanisms of toxoplasmosis.15
Macroscopic lesions in intermediate hosts infected by Toxoplasma gondii are variable and include splenomegaly and disseminated white foci of necrosis in liver and lung. Additional lesions may be found in myocardium and lymph nodes. In the liver toxoplasmosis is characterized histologically by foci of coagulative necrosis with less inflammatory infiltration.8,14 Tachyzoites of Toxoplasma gondii may be present in hepatocytes and Kupffer cells. Special stains (Giemsa, Ziehl-Neelsen, PAS) can be applied but immunohistochemistry has been proven to detect the parasites easily within the tissue.14 Differential diagnoses for toxoplasmosis are neosporosis and sarcosporidiosis, but the lesions, the tissue distribution as well as the affected species, are quite different.14
In humans, Toxoplasma gondii can cause abortion and stillbirth or severe neurologic and/or ocular disease in the fetus during pregnancy. The main routes of infection in man are ingestion of oocyst-contaminated soil and water or eating undercooked meat containing cysts. Other modes of transmission are less common. Most people infected after birth are asymptomatic, some may develop fever, malaise and lymphadenopathy. In immunocompromised individuals, overt disease may develop due to T cell deficiencies.6,15 Interestingly, in human medicine, a coincidence between cerebral toxoplasmosis and mood disorders, schizophrenia, psychoses, depression or suicide and many other diseases and syndromes have been discussed.7
JPC Diagnosis:
2. Liver: Cholangitis, lymphoplasmacytic, multifocal, moderate, subacute, with biliary hyperplasia and intraepithelial coccidian oocysts.
Conference Comment:
In addition to the differential diagnoses of neosporosis and sarcosporidiosis mentioned by the contributor, another apicomplexan protozoan parasite of European moles that should be considered is Elleipsisoma thomsoni. This intraerythrocytic protozoan commonly encysts in the lungs and heart, but occasionally affects the liver, spleen, and kidneys.11 However, demonstration of protozoal tachyzoites and cysts associated with coagulative necrosis in the liver is highly suggestive of T. gondii infection in any homeothermic species.12 In this case, the contributor also demonstrated T. gondii antigen within hepatocytes via immuno-histochemistry.
T. gondi is capable of infecting numerous cell types and its intracellular growth and replication causes eventual cell death. Attachment of the parasite to the cell occurs via the parasite major surface proteins (SAG-1, P30) which are expressed in abundance on tachyzoites. The protozoan also binds extracellular laminin to its surface and then attaches to laminin receptors on host cells. The specialized apicomplexan club-shaped secretory rhoptry organelle then secretes lytic enzyme facilitate cell penetration.4,16
The key feature of the pathogenesis of T. gondiiis its ability to cross multiple types of barrier systems such as the intestinal mucosa, blood-brain barrier, blood-retinal barrier, and placenta by infecting endothelial cells causing vasculitis and ischemic necrosis. In addition, T. gondiiavoids detection by the immune system by forming a parasitophorous vacuole within the host cell. The parasitophorous vacuole allows the parasite to develop while protected from the phagolysosomes of the host cell. Immuno-suppression of latently infected hosts allows cysts to rupture with reactivation of acute disease.14,16
Conference participants also identified coccidian oocysts within the biliary epithelium as a separate etiology from T. gondii. Cyclospora talpae is a very common extra-intestinal coccidian apicomplexan found within the biliary epithelium in wild European moles.5 Participants noted moderate numbers of male microgamonts and female macrogamonts in the bile duct epithelium. As mentioned by the contributor, this coccidian parasite shares many similarities to Eimeria stiedae in rabbits.8 Readers are encouraged to review Case 3 from this conference for a review of the epidemiology and pathogenesis of E. stiedae in a young immunosuppressed rabbit.
References: