Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 7
October 18th, 2017
CASE I: F1753191 (JPC 4101076).
Signalment: 9-year-old, female intact, Rock Alpine goat, Capra aegagrus hircus, caprine.
History: A 9-year-old, female intact Rock Alpine goat presented to Colorado State University Veterinary Teaching Hospital two months prior to necropsy with a three-day history of hyporexia and lethargy which had progressed to lateral recumbency and complete anorexia. The referring veterinarian had previously diagnosed the doe with louse infestation, endoparasites and a heart murmur. Bloodwork by the referring veterinarian revealed a regenerative anemia, stress leukogram and hypoproteinemia characterized by hypoalbuminemia and the goat was treated with ivermectin. Bloodwork at CSU revealed hyperglycemia and elevated creatinine, creatine kinase and aspartate aminotransferase levels. A fecal floatation revealed heavy loads of coccidia, strongyles and Trichuris spp. During a nine day hospitalization, the doe was treated with intravenous fluids, kaopectate, thiamine, fenbendazole, sulfadimethoxine, oxytetracycline and multiple blood transfusions. After significant improvement of her clinical signs and bloodwork, including partial resolution of the dermatitis, the doe was discharged.
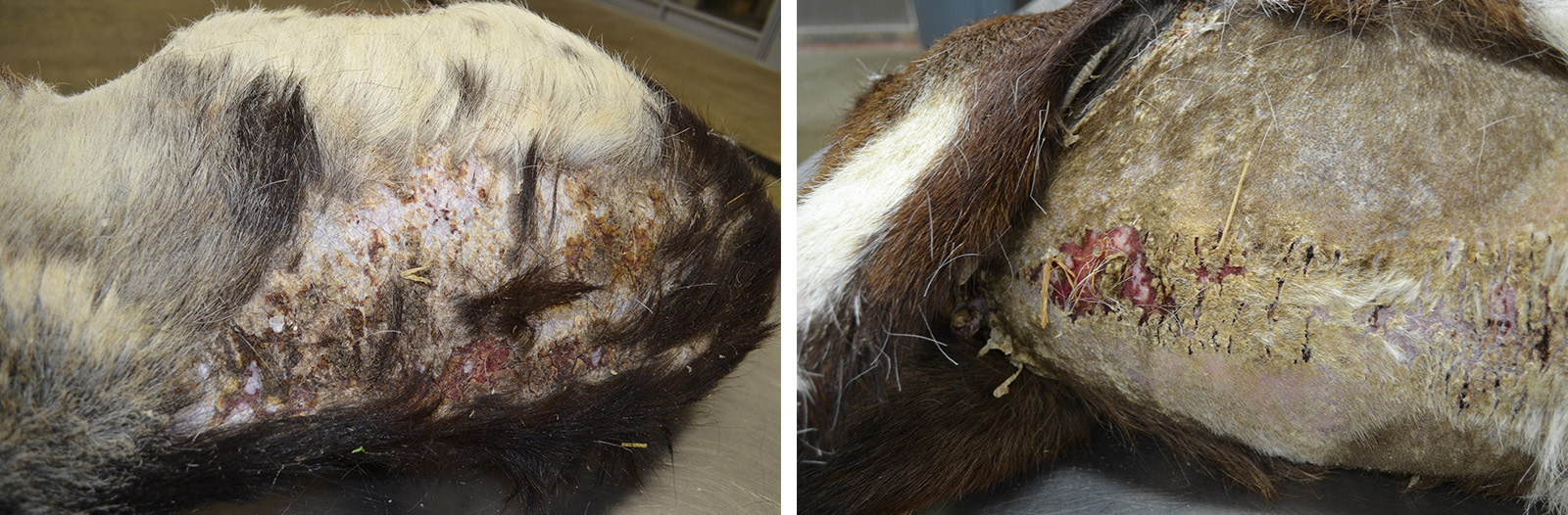
Two months later, the goat presented with a one month history of progressive scaling and ulceration over the withers, dew claws, and coronary bands and acutely progressive lethargy. On physical exam, the doe was febrile, tachypneic and tachycardic. Thoracic radiographs showed a large space-occupying mass which filled the cranial mediastinum and caudally displaced the heart. The doe appeared to be significantly painful, exhibiting shifting leg lameness and refusing to lie down. Humane euthanasia was elected.
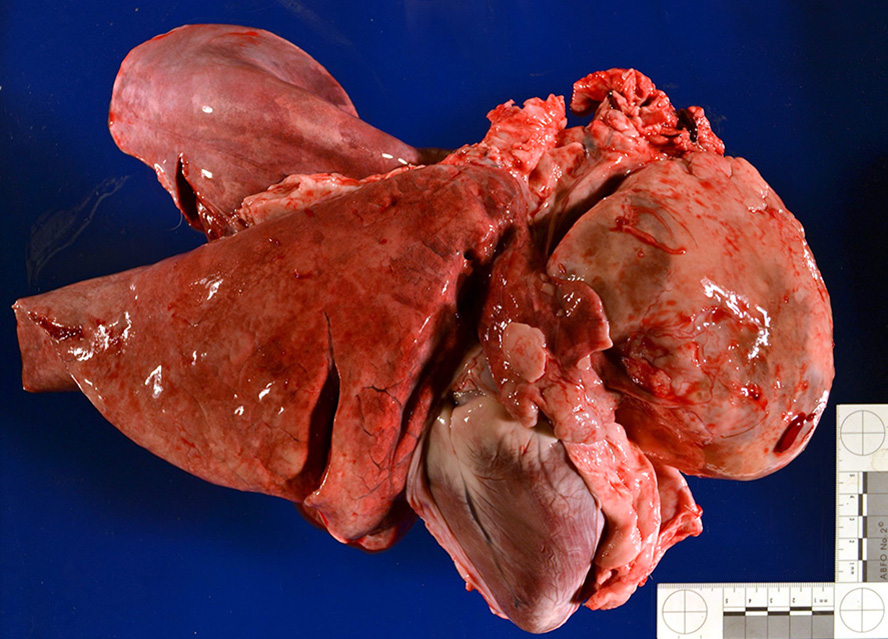
Gross Pathology: Presented for postmortem examination was the carcass of a 9-year-old, female intact Alpine goat in good body condition with mild autolysis. Approximately 60% of the skin was markedly dry, thickened and alopecic with exfoliating epithelial crusts which were often tangled within scant remaining hairs. This lesion most severely affected the skin over the epaxials, the ventral abdomen and teats, coronary bands and dew claws. Lesions were multifocally ulcerated with a reddened hemorrhagic underlying dermis. Occupying approximately 40% of the thoracic cavity was a large 12x8x8cm white, multilobular and cystic mass located cranial to the heart within the mediastinal space. An 8 cm in diameter cystic cavity in the mass was filled with translucent yellow fluid. The heart was caudally displaced and the pericardial sac contained approximately 200 mL of serous fluid. Small strands of fibrin were attached to regions of the pleura and pericardial sac which were in contact with the mass. All other organ systems were grossly within normal limits.
Laboratory results (clinical pathology, microbiology, PCR, ELISA, etc.): Bloodwork was performed at CSU during the does second hospitalization.
Initial PCV was 30% with a total protein of 6.5 G/dl. CBC showed neutrophilia (12.2; 2-6 x 103/ul) and no ongoing evidence of anemia. Chemistry showed hypoalbuminemia (2.2; 3.3-4.2 g/dL), mild hyperglobulinemia (5.0; 3.4-4.8 g/dL), hyperglycemia (207; 45-75 mG/dL), hypomagnesemia (1.4; 2.2-2.9 mg/dL), mild hypokalemia (3.67; 3.8-6.3mEQ/L), mild hypochloremia (105.0; 109-117 mEQ/L) and hypoferremia (51; 110-200 uG/dL)
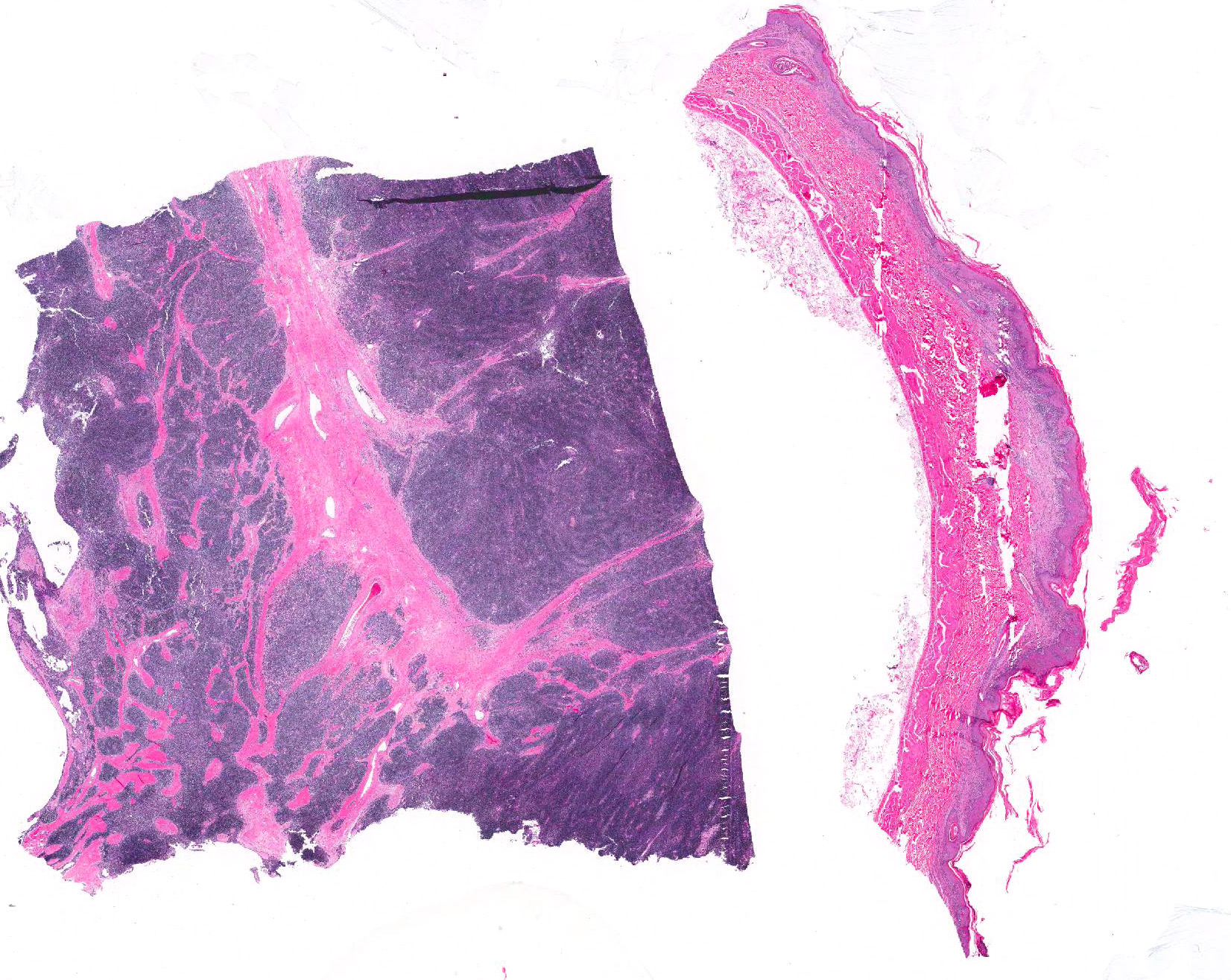
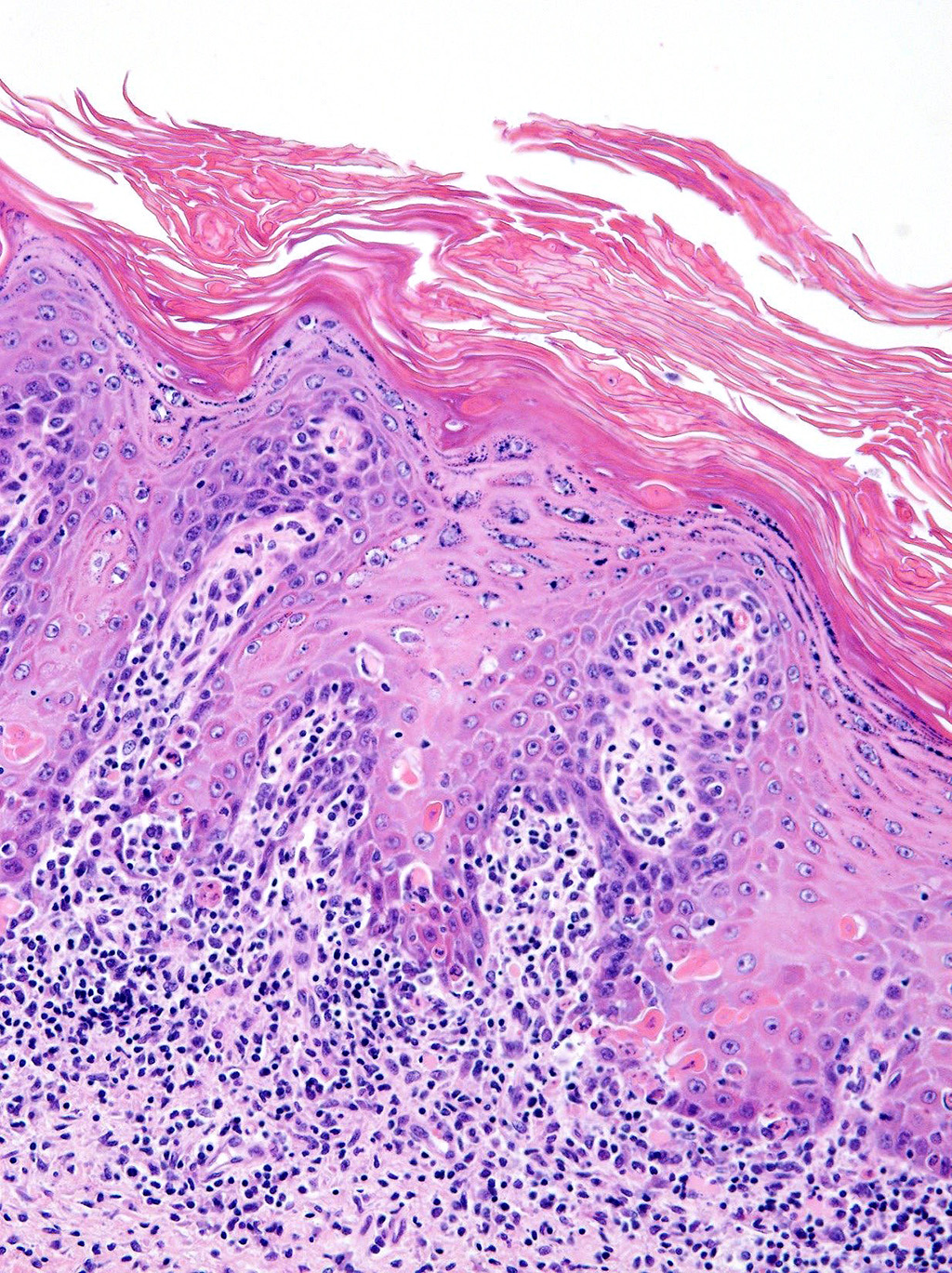
Microscopic Description: Haired skin: The epidermal-dermal junction and perifollicular interstitium are multifocally infiltrated by moderate numbers of lymphocytes. The superficial dermis is expanded by a moderately dense band of lymphocytes with occasional plasma cells, macrophages and neutrophils. Low numbers of mixed inflammatory cells (lymphocytes, plasma cells, neutrophils and macrophages) are present in the dermis. The epidermis is multifocally acanthotic with orthokeratotic and parakeratotic hyperkeratosis. Folliculosebaceous units are decreased in number (variable in sections). Remaining units are variably atrophied. Individual keratinocytes and basal cells and rare follicular epithelial cells are shrunken with hypereosinophilic cytoplasm and pyknotic or lost nuclei and there is occasional satellitosis. Basal cells are occasionally swollen with abundant vacuolated cytoplasm. There is multifocal ulceration and a thick crust composed of numerous degenerate neutrophils, hair shaft fragments and keratin flakes admixed with abundant eosinophilic debris and occasional serum lakes. Scattered throughout this crust are numerous colonies of 1-3 micron cocci, 2-4 micron long asymmetrically peanut-shaped budding yeasts and rare ruminal contents.
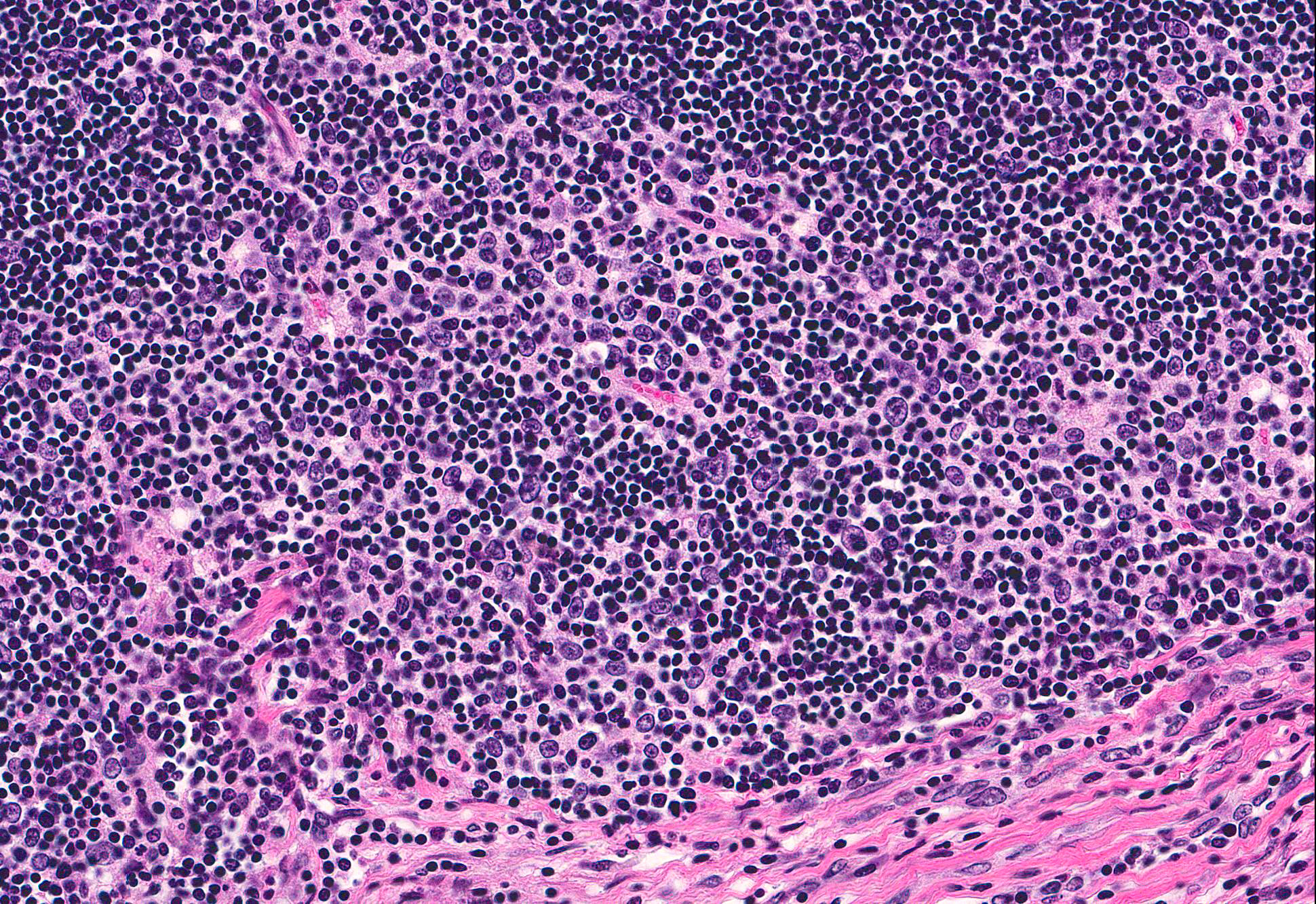
Cranial mediastinal mass: Examined is an encapsulated, highly cellular neoplasm. The neoplasm is composed of loose cords of large polygonal epithelial cells supported by a delicate fibrovascular stroma. Cells have distinct cell borders and abundant eosinophilic cytoplasm. Cell nuclei are ovoid with finely stippled chromatin and indistinct nucleoli. Anisocytosis and anisokaryosis are marked. Mitoses are rare. The neoplasm is infiltrated and frequently obscured by sheets of numerous small mature lymphocytes.
Immunohistochemistry for cytokeratin, CD3, and CD79a was performed on the cranial mediastinal mass. Neoplastic epithelial cells demonstrated strong, diffuse cytoplasmic immunoreactivity for cytokeratin. Approximately 95% of lymphocytes infiltrating the mass are CD3 immunoreactive. Rare infiltrating lymphocytes are immunoreactive for CD79a within the cytoplasm.
In serial sections of skin, a GMS preparation highlighted superficial argyrophilic asymmetric 2-4 micron long, peanut-shaped yeasts and a Gram stain highlighted 1-3umgram positive cocci.
Contributors Morphologic Diagnoses>:
1. Haired skin: Dermatitis and folliculitis, interface, lymphocytic, chronic active, severe with rare keratinocyte, basal cell and follicular epithelial cell apoptosis and superficial cocci and yeasts.
2. Cranial mediastinal mass: Thymoma, lymphoepithelial (mixed).
Name of Disease: Thymoma-associated exfoliative dermatitis
Contributors Comment: Thymomas arise from the epithelial components of the thymus. They are classified as epithelial, lymphocytic or lymphoepithelial (mixed), based on the degree of infiltration by non-neoplastic lymphocytes. In one survey of 102 tumors in goats, thymomas represented the third most common tumor.6 Dairy breeds may be predisposed.5 These tumors tend to be benign, although there is a report of thymic carcinoma in one goat with metastases to the lung and spleen.7 Frequently thymomas are an incidental finding in goats with no associated clinical signs; however, reported sequelae include congestive heart failure and megaesophagus.8,9
Thymoma-associated exfoliative dermatitis is an established paraneoplastic syndrome of cats.1, 4, 10, 11 The syndrome has also been reported in rabbits.3 It has been posited that the mechanism of the dermatologic lesion is rooted in the tumor-supported development of a population of autoreactive T cells which target keratinocytes.4 The classic feline cutaneous lesion has been previously described as a cellpoor interface dermatitis with telogenization of follicles.4 However, in a case series of five cats as well as this goat, the lesion is significantly cell-rich, forming large bands of inflammation at the epidermal-dermal interface.10 Other paraneoplastic syndromes which have been associated with thymomas include myasthenia gravis, polymyositis and granulocytopenia.11
Gross lesions in this case were quite striking. Extensive regions of alopecia, erythema and ulceration with large exfoliative flakes affected the dorsum, ventrum and even the teats and dew claws. Histologically, the lesion is characterized by transepidermal and follicular apoptosis, interface dermatitis and hyperkeratosis. The inflammatory infiltrates are composed of lymphocytes, plasma cells, macrophages and superficially located neutrophils. In some cases, sebaceous glands are lost. The lesion can be quite subtle; however, in this case there are regions which are severely affected. The presence of cocci and yeasts consistent with Malassezia spp. varied between sections of the tissue. Secondary infection with bacteria and yeasts can exacerbate the dermatitis and induce pruritus.2
Given the prevalence of thymomas in goats and the frequent lack of directly associated clinical signs, this case is of diagnostic interest for dermatologic lesions in goats. As thymoma-associated exfoliative dermatitis can be histologically similar to erythema multiforme and lupus erythematosus, diagnosis is contingent on the clinical diagnosis of a thymoma. Interestingly, post-thymectomy resolution of the dermatologic lesion has been reported in some cases in cats.1, 2
JPC Diagnosis: 1. Thymus: Thymoma (lymphocytic type), Rock Alpine goat (Capra aegagrus hircus), caprine.
2. Skin: Dermatitis, lymphocytic, interface, diffuse, mild to moderate with multifocal epithelial hyperplasia, keratinocyte apoptosis, intracorneal pustules, and orthokeratotic and parakeratotic hyperkeratosis.
Conference Comment: Paraneoplastic syndromes are systemic conditions caused most commonly by excessive production of a normal hormone by neoplastic cells. The clinical signs they produce are often quite remarkable and are usually the reason the animal is brought to the veterinarian. Clinically, they serve as convenient markers for diagnosticians, and may serve to indicate neoplastic response to treatment. One or more of the following criteria must be met in order to classify a clinical syndrome as paraneoplastic: (1) when the neoplasm is removed or treated, the concentration of the hormone decreases, (2) after removal of the normal gland producing the hormone, the concentration remains the same or increases, (3) there is a positive arteriovenous concentration of the hormone across the tumor, (4) there is production and secretion of the hormone product in vitro. In veterinary literature, the first criterion is most common.
There are several proposed pathogenic mechanisms of paraneoplastic syndromes including: (1) gene de-repression, resulting in the production of active hormones that are usually repressed; (2) ectopic receptor production by a tumor, resulting in displaced hormonal activity (an example is acetylcholine receptors produced by thymomas resulting in anti-acetylcholine receptor antibodies produced by the host immune system, leading to muscle weakness and megaesophagus that characterizes myasthenia gravis); (3) exposure to normally hidden substances which the immune system perceives as foreign andmounts a type III hypersensitivity reaction against, with formation of immune complexes. Cachexia is the most common paraneoplastic response and occurs secondary to any neoplastic process because it is caused by rapid tumor growth and utilization of nutrients at the expense of the animal. It is hypothesized to be related the effects of the following pro-inflammatory mediators: tumor necrosis factor (TNF), interleukins 1 and 6 (IL-1 and IL-6) and interferon gamma and alpha (IFN? and IFN?).2
Table 1: Common paraneoplastic syndromes in veterinary species2, 15
|
Paraneoplastic syndrome |
Associated neoplasm |
|
Endocrine |
|
|
Hypercalcemia of malignancy |
Lymphoma Apocrine gland carcinoma of the anal sac gland Mammary carcinoma Thymoma |
|
Hypoglycemia |
Hepatocellular carcinoma Salivary gland carcinoma Leiomyoma/leiomyosarcoma Plasma cell tumor Lymphoma |
|
Ectopic ACTH |
Pulmonary carcinoma |
|
Cutaneous |
|
|
Pemphigus |
Lymphoma |
|
Alopecia |
Pancreatic carcinoma (cat) |
|
Exfoliative dermatitis |
Thymoma (cat, rabbit) |
|
Necrolytic migratory erythema |
Glucagonoma |
|
Hematologic |
|
|
Hypergammaglobulinemia |
Multiple myeloma Lymphoma |
|
Anemia |
Numerous neoplasms |
|
Erythrocytosis |
Renal carcinoma |
|
Neurologic |
|
|
Myasthenia gravis |
Thymoma |
|
Peripheral neuropathy |
Insulinoma |
|
Renal |
|
|
Glomerulonephritis |
Multiple myeloma Polycythemia vera |
|
Gastrointestinal |
|
|
Gastroduodenal ulceration |
Mast cell tumors Gastrinoma |
|
Miscellaneous |
|
|
Hypertrophic osteopathy |
Pulmonary carcinoma Other thoracic masses Urinary bladder rhabdomyosarcoma |
|
Cachexia |
Numerous neoplasms |
Thymomas have been rarely reported in most domestic animal species and present as a lobulated mass in the cranial mediastinum in adult to older animals and commonly replace one lobe with thymic remnant compressed at the periphery. In addition to the classification based on cell type and atypia listed above (epithelial, lymphocytic, or mixed) a more accurate classification has been based on two main benign phenotypes: type A (composed of spindle shaped cells) and type B (composed of epithelioid cells). Type B thymomas are more common in dogs and are further subdivided into three subtypes: (1) type B1 which resembles normal thymus with extensive lymphocyte proliferation (may be mistaken for a lymphoid neoplasm), (2) type B2 with neoplastic epithelial cells that are more plump, with vesiculate nuclei that are more easily discernible on a dense background of lymphocytes, and (3) type B3 which contain large sheets of neoplastic epithelial cells and very few lymphocytes. Type AB thymomas are plausibly a mix of the two with both epithelial neoplastic cells and lymphocytes mixed together. In goats and sheep, type AB are most common and present as striking space occupying masses in older females which are often incidental findings during necropsy.16 Clinical signs associated with thymomas include respiratory distress and ventral head and neck edema as well as several paraneoplastic conditions often associated with some form of autoimmunity. The most common is myasthenia gravis which results in generalized muscle weakness and megaesophagus (mechanism described above). Secondary neoplasias such as osteosarcoma and mammary tumors, immune-mediated skin diseases, hypercalcemia, and polymyositis have also been reported.15 Hypercalcemia is due to production of PTHrp which results in increased osteoclastic activity and increased calcium reabsorption in the proximal and distal convoluted tubules of the kidney.11 In human medicine, the mechanism of increased autoimmunity has been worked out and is explained in the following sentences. The thymus, under normal conditions, plays a central part in the development of immunity and prevention of autoimmunity. Thymic epithelial cells express MHC I and MHC II antigens that react with circulating T-lymphocytes. Thymomas enhance thymic lymphopoiesis; expression of autoantigens and reduced expression of MHC molecules and autoimmune regulator molecules (AIRE) on neoplastic epithelial cells results in unreliable negative selection and release of autoreactive T lymphocytes.15
For the lesions in the skin, conference participants also considered erythema multiforme (EM) which is an autoimmune skin disorder that has been anecdotally reported in the goat. EM has been reported in association with a number of disorders, including: adverse drug reactions, infectious diseases (parvovirus infection in dogs, Equid herpesvirus-5, feline herpesviral infections), and in cats, thymoma-associated exfoliative dermatitis has been equated to EM in the literature. Grossly, EM presents as erythematous papules and plaques with a central area of clearing. The classic microscopic presentation is cytotoxic (interface) dermatitis with necrotic keratinocytes scattered throughout all layers of the epidermis and follicular epithelium often surrounded by lymphocytes (satellitosis).8
Colorado State University
Microbiology, Immunology, and Pathology Department
College of Veterinary Medicine and Biomedical Sciences http://csucvmbs.colostate.edu/academics/mip/Pages/default.aspx
References:
1. Cavalcanti J, Moura M, Monteiro F. Thymoma associated with exfoliative dermatitis in a cat. J Feline Med Surg. 2014;16(12):1020-1030.
2. Cullen JM, Breen M. An overview of molecular cancer pathogenesis, prognosis, and diagnosis. In: Meuten DJ, ed. Tumors of Domestic Animals. 5th ed. Ames, IA: John Wiley & Sons, Inc.; 2017:15-16.
3. Forster-Van Hijfte MA, Curtis CF, White RN. Resolution of exfoliative dermatitis and Malassezia pachydermatis overgrowth in a cat after surgical thymoma resection. J Small Anim Pract. 1997;38(10):451-454.
4. Florizoone K. Thymoma-associated exfoliative dermatitis in a rabbit. Vet Dermatol. 2005;16(4):281-284.
5. Gross TL. Ihrke PJ. Walder EJ, et al. Skin Diseases of the Dog and Cat. 2nd ed. Ames, IA: Blackwell Science Ltd; 2005:68-70, 78-79
6. Hadlow WJ. High prevalence of thymoma in the dairy goat report of seventeen cases. Vet Pathol. 1978;15:153-169.
7. Löhr C V. One hundred two tumors in 100 goats. Vet Pathol. 2012;50(4):668-675.
8. Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol. 1. 6th ed. St. Louis, MO: Elsevier; 2016:609-610.
9. Olchowy T, Toal R, Brenneman K, Slauson D, McEntee M. Metastatic thymoma in a goat. Can Vet J. 1996;37(3):165-167.
10. Parish SM, Middleton JR, Baldwin TJ. Clinical megaoesophagus in a goat with thymoma. Vet Rec. 1996;139:94.
11. Rosol TJ, Meuten DJ. Tumors of the endocrine glands. In: Meuten DJ, ed. Tumors of Domestic Animals. 5th ed. Ames, IA: John Wiley & Sons, Inc.; 2017:816-821.
12. Rostkowski CM, Stirtzinger T, Baird JD. Congestive heart failure associated with thymoma in two nubian goats. Can Vet J. 1985;26:267-269.
13. Rottenberg S, Tscharner C Von, Roosje PJ. Thymoma-associated exfoliative dermatitis in cats. Vet Pathol. 2004;41(4):429-433.
14. Singh A, Boston SE, Poma R. Thymoma-associated exfoliative dermatitis with post-thymectomy myasthenia gravis in a cat. Can Vet J. 2010;51.
15. Valli VE, Bienzle D, Meuten DJ. In: Meuten DJ, ed. Tumors of Domestic Animals. 5th ed. Ames, IA: John Wiley & Sons, Inc.; 2017:305-307.
16. Valli VEO, Kiupel M, Bienzle D, Wood RD. Hematopoietic system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol. 3. 6th ed. St. Louis, MO: Elsevier; 2016:151-158.