Results
AFIP Wednesday Slide Conference - No. 23
March 15 2000
- Conference Moderator:
Dr. Wallace B. Baze, Diplomate, ACVP
The University of Texas MD Anderson Cancer Center
Science Park, Department of Veterinary Sciences
Bastrop, Texas 78602
- Return to WSC Case Menu
-
- Case I - 97055 (AFIP2694673)
-
- Signalment: Eight-year-old male rhesus monkey (Macaca
mulatta)
-
- History: Animal died following a six-day illness consisting
of progressive depression, anorexia, labored abdominal breathing,
coughing, and tachypnea.
 a
a
- Case 23-1. Chest radiograph. Diffusely lung fields
are infltrated by flocculent fluid densities.
 b
b c
c
 d
d
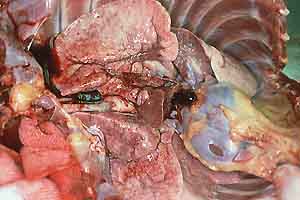
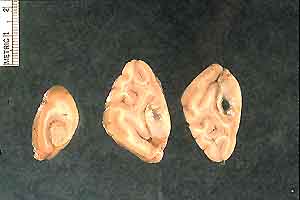
- Case 23-1. Gross Images. Lungs (b) have a pale tan
mottling. Cut section of the fixed lung (c) contains diffusely
distributed pale tan 2-3mm foci. Within the white matter of the
brain (d) is an 0.3x1.0cm cavitating lesion.
-
- Gross Pathology: At necropsy the carcass appeared
in good condition. There was moderate scrotal and inguinal edema.
The lungs were diffusely hyperinflated and did not collapse when
the thoracic cavity was opened. The lungs were gray to white
and had pleural vascular distension and a granular texture throughout
with disseminated firm nodules, ranging in diameter from 1 to
5 mm. The right middle lung lobe was firm and dark red. The spleen
contained a 1 cm in diameter firm, pale-yellow irregular nodule.
There was minimal, focal, gastric ulceration and hemorrhage.
The right cerebral frontal lobe and left occipital lobe each
had 1 cm x 2 cm x 2 cm abscesses.
-
- Gross Morphologic Diagnoses:
1. Severe multifocal (miliary) pyogranulomatous pneumonia
2. Severe multifocal pyogranulomatous encephalitis
3. Mild focal pyogranulomatous splenitis
4. Moderate locally extensive subcutaneous edema
5. Minimal focal acute gastric hemorrhage
-
- Laboratory Results: Serum collected two days prior
to the monkey's death was submitted for Blastomyces dermatitidis
titer; the results were 1:32, with greater than 1:8 considered
positive.
-
- Contributor's Diagnosis and Comments: Severe chronic
pyogranulomatous encephalitis with intralesional fungal organisms
(consistent with Blastomyces dermatitidis).
-
- Other Histologic Diagnoses (slides not submitted):
1. Severe, multifocal, pyogranulomatous pneumonia
2. Mild, pyogranulomatous tracheobronchial lymphadenitis
3. Moderate, focal pyogranulomatous splenitis
4. Minimal focal granulomatous hepatitis
-
- All pyogranulomatous lesions contained intralesional fungal
organisms consistent with Blastomyces dermatitidis.
-
- Etiology: Blastomyces dermatitidis
-
- This animal was given an intravenous injection of latex microspheres
for experimental purposes, shortly before its illness became
clinically apparent. In some slides, microspheres are evident;
they appear as refractile, pale yellow, round structures, approximately
30 microns in diameter.
-
- Blastomycosis has not been reported previously in nonhuman
primates. The acute onset of respiratory signs following the
experimental procedure confounded ante-mortem diagnosis. Also
confounding diagnosis were the disease's previously unrecognized
status in nonhuman primates and the animal's lack of exposure
to soil while at our facility from June 1996 until death in February
1997, suggesting the incubation period in this animal was greater
than 4.5 years.
 e
(H&E)
e
(H&E)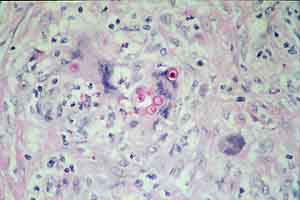 f
(PAS)
f
(PAS)
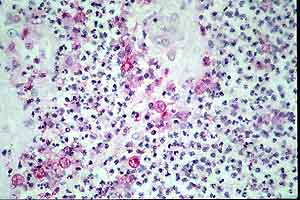 g
(PAS)
g
(PAS)
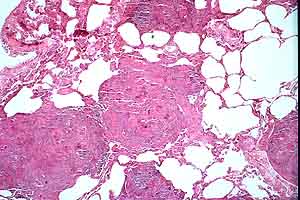
- Case 23-1. Multifocally within the lung, there are
fibrotic foci (e,f) that contain neutrophils (f,g), epithelioid
cells (f,g), occasional foreign body giant cells (e,f,g), and
scattered 8-12u diameter yeast bodies (f,g).
-
- AFIP Diagnosis: Cerebrum: Abscesses and pyogranulomas,
multifocal, with budding yeast, rhesus monkey (Macaca mulatta),
nonhuman primate, etiology consistent with Blastomyces dermatitidis.
Conference Note: Blastomycosis is primarily a disease
of humans and dogs but also is seen in other animals, including
the cat, horse, sea lion, wolf, ferret, dolphin and polar bear.
The disease occurs primarily in the Mississippi-Ohio river basins,
the central U.S. Atlantic states, and in the northern border
of Ontario and Manitoba in Canada. Blastomyces dermatitidis,
the causative agent of North American blastomycosis, is a dimorphic
fungus that produces a mycelial growth at room temperature and
yeast-like forms in tissue and in culture at 37 degrees Celsius.
-
- The organism reproduces by budding and can be found free
or within macrophages in affected tissues. Although the lung
is considered the most common site of primary involvement, primary
cutaneous infections, and disseminated disease can occur. Disseminated
disease most commonly affects the lung, lymph nodes, skin, eyes,
bone, joints, and subcutaneous tissues. Occasionally, primary
cutaneous infection may occur from a puncture wound in the skin.
However, because skin lesions more commonly result from disseminated
infection, cutaneous blastomycosis should arouse suspicion of
systemic disease. Pulmonary lesions are sometimes resolved by
the time the sites of disseminated infection become apparent.
-
- The differential diagnosis discussed during the conference
included cryptococcosis, histoplasmosis, aspergillosis, coccidioidomycosis,
African histoplasmosis (Histoplasma capsulatum var. duboisii)
and South American blastomycosis (Paracoccidioides braziliensis).
Cryptococcus neoformans is characterized by a wide, carminophilic
capsule. Histoplasma capsulatum is much smaller than Blastomyces,
and has narrow-based budding. Aspergillus sp. often present
as narrow, septate hyphae that branch dichotomously at acute
angles. Coccidioides immitis is larger, and reproduces
by endosporulation rather than budding. Histoplasma capsulatum
var. duboisii has similar size and shape to Blastomyces
dermatitidis, but the former has uninucleate yeast-like cells
narrower based buds while the latter has multinucleate yeast-like
cells and broad based buds. South American blastomycosis reproduces
in tissue by multiple budding.
-
- Contributor: Wake Forest University School of Medicine,
Department of Pathology, Section on Comparative Medicine, Medical
Center Boulevard, Winston-Salem, NC 27157-1040
-
- References:
- 1. Cote E, Barr SC, Allen C, Eaglefeather E: Blastomycosis
in six dogs in New York State. J Amer Vet Med Assoc 210(4):502-504,
1997
- 2. Dungworth DL: The respiratory system. In: Pathology of
Domestic Animals, eds. Jubb KVF, Kennedy PC, Palmer N, 4th ed.,
vol. 2, pp. 667, Academic Press, San Diego CA, 1993
- 3. Jones TC, Hunt RD, King NW: Diseases caused by fungi.
In: Veterinary Pathology, 6th ed., pp. 505-547, Williams and
Wilkins, Baltimore MD, 1997
- 4. Legendre AM: Blastomycosis. In: Infectious Diseases of
the Dog and Cat, 2nd ed., pp. 371-377, WB Saunders Co, Philadelphia,
PA, 1998
- 5. Wilkinson LM, Wallace JM, and Cline JM: Disseminated blastomycosis
in a rhesus macaque (Macaca mulatta). Vet Pathol 36(5):460-462,
1999
-
-
- Case II - MC99-7 ( AFIP 2694669)
-
- Signalment: 2½-year-old male rhesus monkey
(Macaca mulatta)
-
- History: This monkey was humanely sacrificed due to
persistent diarrhea, significant weight loss (>20%), and a
generally poor condition.
-
- Gross Pathology: The lungs were relatively firm and
poorly collapsible, with multiple patchy foci of congestion and
hemorrhage.
Contributor's Diagnoses and Comments:
1. Interstitial pneumonitis, subacute, patchy, moderate.
2. Syncytial giant cell infiltrate, primarily alveolar, patchy,
moderate-marked.
3. Amorphous, foamy to granular amphophilic alveolar material
consistent with Pneumocystis (most sections).
4. Alveolar proteinosis & fibrin exudation, patchy, mild.
5. Angitis, subacute, multifocal, mild.
-
- This monkey had been inoculated IV 9 months previously with
SIV/Delta B670. Most of the microscopic findings are characteristic
for, and the direct result of, lentivirus (SIV) infection. These
lesions, especially the distinctive syncytial cell formation
(in which viral antigens and particles are demonstrable) are
not typical of lentivirus infection in sheep, goats or humans
with HIV. Pneumocystis carinii is an important opportunistic
respiratory pathogen the environmental source of which is not
always known. It is commonly seen in conjunction with the immunosuppression
created by SIV and is noted with significant frequency (>50%)
during later stages of macaque lentivirus infection.
-
- The lentivirus (SIV) pulmonary infection was confirmed postmortem
by immunohistochemistry and Pneumocystis organisms were visualized
by GMS staining and also confirmed by immunohistochemical evaluation.
-
- AFIP Diagnoses:
- 1. Lung: Pneumonia, interstitial, lymphoplasmacytic and histiocytic,
diffuse, mild, with syncytial cells and edema, rhesus monkey
(Macaca mulatta), nonhuman primate.
2. Lung: Pneumonia, interstitial, lymphohistiocytic, mild, with
intraalveolar foamy material (atypical fungi), etiology consistent
with Pneumocystis carinii.
Conference Note: Simian immunodeficiency virus (SIV) is
a member of the Lentivirus genus in the family Retroviridae along
with human immunodeficiency virus, equine infectious anemia virus,
ovine lentivirus, bovine immunodeficiency-like virus, and feline
immunodeficiency virus. In addition to the lungs, other organs
commonly affected by SIV include brain, lymph nodes, thymus,
gastrointestinal tract, and skin. Secondary infections are common
with SIV infection and include pneumocystosis, cryptococcosis,
toxoplasmosis, candidiasis, mycobacteriosis, nocardiosis, disseminated
salmonellosis, and infection with cytomegalovirus, herpes simplex
virus, and rhesus gamma herpesvirus.
Pneumocystis carinii is an atypical fungus that is ubiquitous
in the environment and can frequently be found in normal lungs
without associated lesions. P. carinii is often the first opportunistic
infection diagnosed in HIV-1 infected humans, and is the leading
cause of death in AIDS.
SIV and the secondary infections that often accompany it are
very similar to the situation with human AIDS, making the macaque-SIV
model an extremely valuable tool for the study of HIV and AIDS.
-
- Contributor: Division of Laboratory Animal, S-1040
BioMedical Science Tower, University of Pittsburgh, PA, 15261
-
- References:
- 1. Baskerville A, Dowsett AB, Cook RW, Dennis MJ, Cranage
MP, Greenaway PJ: Interstitial pneumonia in simian immunodeficiency
virus infection. J Pathol 167:241-247, 1992
- 2. Baskerville A, Dowsett AB, Cook RW, Dennis MJ, Cranage
MP, Greenaway PJ: Pneumocystis carinii pneumonia in simian immunodeficiency
virus infection: Immunohistological and scanning and transmission
electron microscopical studies. J Pathol 164:175-184, 1991
- 3. Baskin GB, Murphey-Corb M, Martin LN, Soike KF, Hu F-S,
Kuebler D: Lentivirus-induced pulmonary lesions in rhesus monkeys
(Macaca mulatta) infected with simian immunodeficiency virus.
Vet Pathol 28:506-513, 1991
- 4. Bennett BT, Abee CR, Henrickson R: Nonhuman Primates in
Biomedical Research - Diseases, pp. 39-43 & 298-299. Academic
Press, New York, 1998
- 5. Cotran RS, Kumar V, Collins T: Pathologic Basis of Disease,
6th ed., pp. 247-248. Saunders, Philadelphia, PA, 1999
- 6. Vogel AP, Miller CJ, Lowenstine LJ, Lackner AA: Evidence
of horizontal transmission of Pneumocystis carinii pneumonia
in simian immunodeficiency virus-infected rhesus monkeys. J Infect
Dis 168:836-843, 1993
-
-
- Case III - S-63504 (AFIP 2715544)
-
- Signalment: 5-year-old, rhesus macaque, female, Macaca
mulatta
-
- History: This domestic purpose-bred macaque was born
in 1993 and had an uneventful history in an AAALAC-accredited
facility until the autumn of 1998, when she was anorexic. Physical
examination revealed hypothermia (96.5(F)), tachypnea (136 breaths/minute),
dehydration, decreased body mass, mild hepatosplenomegaly, and
upper abdominal tenderness. Thoracic auscultation revealed loud
respiratory sounds with labored inspiratory and expiratory components.
Apex heart sounds were shifted to the right.
-
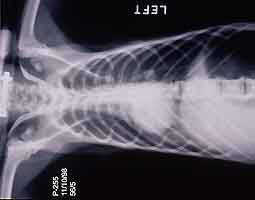
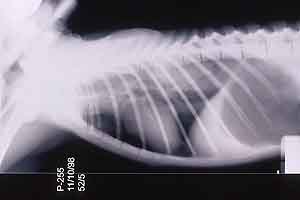
- Case 23-3. The A/P thoracic radiograph shows marked
right sided deviation of the cardiac silouette. The lateral thoracic
radiograph shows diffuse fluid density in dorsal lung fields.
-
- Thoracic radiographs demonstrated an increased pulmonary
interstitial pattern with air bronchograms bilaterally. The cardiac
silhouette was shifted to the right. Abdominal radiographs demonstrated
hepatomegaly and the presence of excessive gas in the stomach,
small intestine, and colon. Supportive treatment, including oral,
subcutaneous, and intravenous fluids, antibiotics, and vitamins
were administered, but response to treatment was minimal and
the monkey was euthanized in late 1998.
-
- Gross Pathology:
- Body weight at necropsy was 5.5 kg. Numerous petechial and
ecchymotic hemorrhages were present in the skin of the forelimbs,
the ventral thorax, ventral abdomen, and hindlimbs. The subcutis
of the ventral abdomen was edematous.
-
- Approximately 100 ml of red fluid and 25 ml of clotted blood
were in the right pleural cavity. The right middle and caudal
lung lobes were dark red to black and firm. On cut surface a
cavitated focus (5x5x10mm) was present in the dorsal aspect of
the right caudal lobe; the surrounding tissue was dark red to
black and friable. The visceral pleura overlying the cavitated
focus was focally adhered to the parietal pleura by means of
a circular, tan, 2 cm diameter plaque. The costal surface of
the remainder of the right caudal lobe and the right middle lobe
were covered with blood and fibrin and diffusely adhered to the
parietal pleura. These two lung lobes were also adhered to each
other, as well as to the diaphragm, pericardial sac, and mediastinum.
-
- Laboratory Results:
- Serial hematology and serum biochemistry samples revealed
a severe leukocytosis (21 to 35 x103/mm3) with mature neutrophilia
(18 to 31 x103/mm3), a developing mild anemia (36%), decreased
platelet count (90 to 172 x103/mm3), and marked increases in
BUN (265 to 411 mg/dl) and creatinine (8.0 to 15.0 mg/dl).
- Pleural fluid was submitted for culture and sensitivity.
Corynebacterium ulcerans was isolated and was reported
sensitive to a variety of antibacterial agents, including cefazolin,
cephalothin, penicillin, erythromycin, gentamicin, tetracycline,
trimethoprim/sulfa, vancomycin, nitrofurantoin, sulfasoxazole,
and enrofloxacin.
- Contributor's Diagnosis and Comments:
Lung: necrotizing bronchopneumonia and fibrinohemorrhagic pleuritis
Etiologic agent: Corynebacterium ulcerans
-
- Corynebacterium ulcerans has been isolated from cattle
with mastitis and cutaneous infections (Hommez et al. 1999; Hart
1984), nonhuman primates with abscesses, bite wounds, pneumonia,
and mastitis (Fox and Frost, 1974; May 1972; Panaitescu et al.
1977), and humans with sore throat, tonsillitis, nasopharyngitis,
laryngitis, and pulmonary nodules (de Carpentier et al. 1992;
Dessau et al. 1995; McDonald et al. 1997; Hust et al. 1994).
Often human infections have been associated with contact with
livestock or ingesting unpasteurized dairy products. Based on
genomic DNA relatedness, Corynebacterium ulcerans is a
distinct species closely related to C. diphtheriae and
C. pseudotuberculosis (biovars equi and ovis) (Riegel
et al. 1995).
-
- Like these related species and biovars, C. ulcerans
produces potent exotoxins, specifically diphtheria toxin and
necrotizing (C. ovis -like) toxin. Diphtheria toxin is
an acidic protein which inhibits protein synthesis by inactivation
of elongation factor 2, while necrotizing (C. ovis -like)
toxin is a basic glycoprotein, a phospholipase D and a sphingomyelinase,
and increases vascular permeability, leading to local edema (Carne
and Onon, 1982).
-
- Based on the known pathogenesis of C. diphtheriae,
it is hypothesized that the inhalation/ingestion of C. ulcerans
is followed by local proliferation on the upper and/or lower
respiratory tract epithelium, release of toxins, necrosis of
the colonized and adjacent epithelium, an intense neutrophilic
infiltrate in the underlying tissue, vascular congestion, interstitial
edema, and fibrinosuppurative exudation.
-
- Corynebacterium ulcerans should be considered in the
differential diagnosis when confronted with a nonhuman primate
with suspected bronchopneumonia and/or pulmonary abscesses. Therapeutic
intervention, if warranted, should include appropriate antimicrobial
therapy, based on bacterial isolation and sensitivity testing,
diphtheria antitoxin, and supportive care.
-
- AFIP Diagnoses:
- 1. Lung: Pneumonia, suppurative and hemorrhagic, multifocal
to coalescing, moderate, with chronic active pleuritis, rhesus
macaque (Macaca mulatta), nonhuman primate.
2. Lung: Pneumonia, fibrinous, subacute to chronic, diffuse,
with chronic fibrinohemorrhagic pleuritis.
Conference Note: Corynebacteria sp. are Gram positive,
generally non-motile, pleomorphic bacilli. With the exception
of C. diphtheriae, they are generally part of the normal
flora and they are widely distributed in the environment. C.
ulcerans is a commensal of horses and cattle.
-
- The differential diagnosis discussed in conference included
Pasteurella sp., Legionella pneumophila, Actinobacillus sp.,
and Corynebacterium sp. A Gram's stain demonstrated small numbers
of pleomorphic Gram positive bacilli within the affected areas.
-
- Contributor: Merck Research Laboratories, Departments
of Safety Assessment and Laboratory Animal Resources, West Point,
PA and Rahway, NJ.
-
- References:
- 1. Carne HR, Onon OE: The exotoxins of Corynebacterium ulcerans.
J Hyg Camb 88:173-191, 1982
- 2. de Carpentier JP, Flanagan PM, Singh IP, Timms MS, Nassar
NY: Nasopharyngeal Corynebacterium ulcerans: a different diptheria.
J Laryn Otolo 106:824-826, 1992
- 3. Dessau RB, Brandt-Christensen M, Jensen OJ, Tonnesen P:
Pulmonary nodules due to Corynebacterium ulcerans. Eur Respir
J 8:651-653, 1995
- 4. Fox JG, Frost WW: Corynebacterium ulcerans mastitis in
a bonnet macaque (Macaca radiata). Lab Anim Sci 24:820-822, 1974
- 5. Hart RJC: Corynebacterium ulcerans in humans and cattle
in North Devon. J Hyg 92:161-164, 1984
- 6. Hommez J, Devriese LA, Vaneechoutte M, Riegel P, Butaye
P, Haesebrouck F: Identification of nonlipophilic Corynebacterium
isolated from dairy cows with mastitis. Journ Clin Micro 37:954-957,
1999
- 7. Hust MH, Metzler B, Schubert U, Weidhase A, Seuffer RH:
Toxische Diphtherie durch Corynebacterium ulcerans. DMW 119:548-552,
1994
- 8. May BD: Corynebacterium ulcerans infections in monkeys.
Lab Anim Sci 22:509-513, 1972
- 9. McDonald S, Cox D, Allen R, Bixler D, Steele G: Respiratory
Diptheria Caused by Corynebacterium ulcerans. Terre Haute, Indiana,
1996. MMWR 46:330-332, 1996
- 10. Panaitescu M, Maximescu P, Michel J, Potorac E: Respiratory
pathogens in non-human primates with special reference to Corynebacterium
ulcerans. Lab Anim 11:155-157, 1977.
11. Riegel P, Ruimy R, de Briel D, Prevost G, Jehl F, Christen
R, Monteil H: Taxonomy of Corynebacterium diphtheriae and related
taxa, with recognition of Corynebacterium ulcerans sp. nov. nom.
rev. FEMS Microbiology Letters 126: 271-276, 1995
-
-
- Case IV - 97-1185 (AFIP 2694774)
-
- Signalment: 6-year-old, American domestic shorthair,
castrated male, feline.
-
- History: Two weeks prior to presentation at the North
Carolina State University Veterinary Teaching Hospital this cat
received anesthesia for dental prophylaxis. Recovery from anesthesia
was prolonged, and the cat became acutely blind and severely
lethargic. Over a 2-week period the cat's appetite decreased,
a right head tilt developed, and the cat become progressively
lethargic. At the veterinary teaching hospital an ophthalmic
examination showed a normal fundus and pupillary light response;
however, the menace reflex was absent. Neurological examination
revealed occasional circling to the right. Titers for Toxoplasma
gondii, Cryptococcus neoformans, feline infectious peritonitis
virus, and feline immunodeficiency virus were negative. The owner
refused further clinical work-up and the cat was euthanized for
necropsy.
- Gross Pathology: No significant lesions.
-
- Laboratory Results: No significant clinical pathology
or microbiology data.
-
- Contributor's Diagnoses and Comments:
- 1. Brain, cerebral cortex and hippocampus: Multifocal, severe,
subacute cerebral cortical necrosis with microcavitation and
gliosis.
2. Brain, hippocampus: Multifocal, mild lymphocytic meningoencephalitis.
-
- Microscopically there are bilateral lesions within the dorsal
gyri of the cranial telencephalon, as well as the dorsal, lateral,
and ventral gyri of the caudal telencephalon. Affected areas
are characterized by neuropil vacuolization, prominent vasculature
with perivascular edema, and moderate gliosis composed of astrocytes
and large foamy microglia. These regions contain low numbers
of ischemic neurons having sharply angular eosinophilic cytoplasm
with pyknotic to karyorrhectic nuclei. In some regions the neuropil
vacuolation often coalesces to form microcavitations. Similar
changes are present within some sections of the hippocampus,
predominantly throughout Ammon's horn, and with less severity
the dentate gyrus.
-
- A variety of etiologies may cause feline cortical neuronal
necrosis. Hypoxia, hypoglycemia, heavy metal toxins, and feline
ischemic encephalopathy have been implicated in acute neuronal
necrosis. There is no corroborating clinical pathology data to
suggest hypoglycemia as an etiology in this cat. Lead inclusions
are usually evident in hematoxylin and eosin stained sections
of brain and kidney; also, there is no history of heavy metal
exposure.
-
- Global brain ischemia, as may occur with disrupted circulatory
conditions, is characterized by neuronal necrosis. With severe
hypotension, the brain's perfusion becomes inadequate causing
bilateral infarcts at the boundary, or watershed zones, between
areas supplied by major arteries. The dorsal gyri are a watershed
zone since the anterior cerebral arteries supply the medially
located cingulate gyri and the middle cerebral arteries supply
the lateral gyri. Sparing of the cingulate and lateral gyri,
and lesions predominantly within the dorsal gyri are characteristic
of those associated with hypotension, and both are present in
this case. The lesions within the lateral and ventral gyri of
the caudal telencephalon, and hippocampus do not correspond with
watershed zones; however, this distribution may reflect a site-specific
sensitivity of these areas to hypoxia.
-
- Humans after cardiac arrest can have necrosis within Ammon's
horn of the hippocampus, and it appears that ischemia selectively
affects the pyramidal neurons of Ammon's horn, while sparing
the hippocampal dentate gyrus. Consistent with ischemia in this
cat is the necrosis within the hippocampal Ammon's horn with
sparing of the dentate gyrus.
-
- The histologic changes observed in this case are suggestive
of global ischemia. There is a syndrome in cats called feline
ischemic encephalopathy (FIE) which can have a histologic presentation
similar to that seen in this cat. FIE has an uncertain etiology,
however, the lesion distribution suggests an underlying vascular
disorder. Cardiomyopathy or aberrant parasite migration by the
larvae of Cuterebra sp. have been suggested as etiologies. FIE
is typically associated with asymmetrical cerebral atrophy in
the lateral gyri, which is the area supplied by the middle cerebral
artery. Cases have been documented with no gross lesions, instead
having histologic evidence of bilateral cerebral cortical and
cerebellar necrosis. Lesions have also been described within
the hippocampus and brain stem, as they also are supplied by
the middle cerebral artery.
-
- The lesions within this cat's dorsal gyri are not a usual
feature of FIE, rather, the dorsal gyri lesions are more characteristic
of global ischemia. Given the cat's history and recent onset
of clinical signs, the brain lesions are probably a direct result
of the anesthetic procedure regardless of any preexisting brain
disease. Furthermore, monitoring systemic blood pressure is unusual
for routine veterinary surgical procedures, thus increasing the
chances for hypotension. It remains possible that pre-existent
subclinical brain disease was exacerbated by the anesthetic procedure.
-
- AFIP Diagnosis: Brain, cerebral cortex and hippocampus:
Necrosis, cortical, laminar, multifocal, with segmental hippocampal
neuronal loss and gliosis, domestic shorthair, feline.
Conference Note: The contributor has provided an excellent
discussion of this case.
-
- Contributor: North Carolina State University, College
of Veterinary Medicine, 4700 Hills Borough St., Raleigh, NC 27606.
- References:
- 1. Auer RN, Siesj BK: Biological differences between ischemia,
hypoglycemia, and epilepsy, Ann Neurology 24:699-707, 1988
- 2. Bernstein NM, Fiske RA: Feline ischemic encephalopathy
in a cat, JAAHA 22:205-206, 1986
- 3. de Lahunta A: Veterinary Neuroanatomy and Clincal Neurology,
2nd edition, pp. 427-429. Philadelphia, WB Saunders Co. 1983
- 4. Garcia JH: The Evolution of Brain Infarcts. A Review,
J Neuropathol Exp Neurol 51:387-393, 1992
- 5. King JM: Feline ischemic encephalopathy, Vet Med 91:1062,
1991
- 6. Palmer AC and Walker RG: The neuropathological effects
of cardiac arrest in animals: a study of five cases, J Small
Anim Pract 11:779-790, 1970.
7. Summers BA, Cummings JF, de Lahunta A: Veterinary Neuropathology,
pp. 237-249. Mosby, Inc., St. Louis, MO, 1995
- 8. Williams KJ, Summers BA, de Lahunta A: Cerebrospinal cuterebriasis
in cats and its association with feline ischemic encephalopathy.
Vet Pathol 35(5): 330-343, 1998
-
-
- J Scot Estep, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: estep@afip.osd.mil
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
-
- Return to WSC Case Menu
 a
a
 b
b c
c
 d
d
 e
(H&E)
e
(H&E) f
(PAS)
f
(PAS)
 g
(PAS)
g
(PAS)

