Results
AFIP Wednesday Slide Conference - No. 15
January 5, 2000
- Conference Moderators:
Dr Richard J Montali and Dr James T Raymond
Department of Pathology
National Zoological Park
- Washington, DC 20008
-
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
- Case I - PV98-1227 (AFIP 2693098)
-
- Signalment: 2½-year-old male chimpanzee (Pan
troglodytes)
-
- History: There was a short course of CNS disease that
abruptly ended a budding movie career. Other chimpanzees in the
gang colony had vague signs of a "cold". This sub-adult
was the only one to develop loss of balance and difficult prehension
with arm pain. There was opisthotonus described but normal cranial
nerve reflexes. The lumbar CSF was clear. Multiple fluffy, cloud-like
areas in the MRI were interpreted as the density of hemorrhages.
-
- Gross Pathology: The brain was mildly swollen in the
calvarium. As depicted in the representative 2x2 slide of formalin-fixed
sections, there were many (>10) random areas of soft purple
discoloration, both in the cortices and brain stem. These spanned
gyri and were easily depressed. Some were partially liquefied
with petechiae and ecchymoses rimming foci in the basal ganglia
and thalamus. There was brown mucus in a bronchus, suggesting
aspiration with subpleural purple foci, but no palpable consolidation.
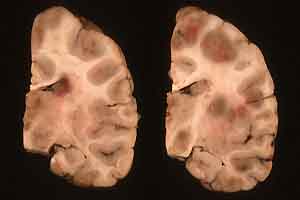
- Case 15-1. Multifocally within the cerebral cortex
and brain stem there are areas of brownish-pink discoloration.
-
- Laboratory Results: Blood cultures negative. The WBC
rose during the week from 23,000 to 35,800. There was mild anemia.
Blood lead levels were normal.
-
- Contributor's Diagnosis and Comments: Multifocal necrotizing
encephalitis, granulomatous, severe, with numerous amoebae.
Etiology: Balamuthia mandrillaris
Sections from all levels of the brain revealed variably severe
to massive malacia and intense inflammation with macrophages,
giant cells and often large numbers of neutrophils. There was
lymphocytic perivascular cuffing with some thrombi and neuronal
necrosis, as well as some foci of parenchymal hemorrhage. The
corpus striatum was especially severely affected with destruction
of the putamen.
-
- Numerous amebic trophozoites are present in the brain but
are often difficult to visualize with H&E, unless the hematoxylin
is well developed. The organisms prove negative with Giemsa,
PAS and silver stains. Mucicarmine was not attempted but is reported
to stain trophozoites.
-
- The diagnosis was confirmed by immunohistochemistry, which
proved positive for Balamuthia and negative for Naegleria and
Acanthamoeba.
There was mild concurrent interstitial pneumonitis and the immunostains
did decorate a few organisms in the septa. This is consistent
with the belief that this pathogen can enter the respiratory
system from inhaled dusts as well as the more usually discussed
rhinocerebral route. Balamuthia is said to be ubiquitous in soil
but has never been isolated from the environment. There is reported
predilection for Old World primates.
-
- AFIP Diagnosis: Brain: Encephalitis, necrotizing,
subacute, multifocal, severe, with necrotizing vasculitis and
amoebae, chimpanzee (Pan troglodytes), nonhuman primate.
-
- Conference Notes: Balamuthia mandrillaris, Acanthamoeba
sp., and Naegleria fowleri are free-living amoebae that have
been reported to cause fatal encephalitis/meningoencephalitis
in humans and animals. B. mandrillaris has been reported in old
world monkeys, sheep, humans and a horse.
In tissues, Balamuthia mandrillaris and Acanthamoeba spp. are
nearly identical. Both appear in two forms: 15-30mm diameter,
round to irregular trophozoites, and rare 10-15mm diameter cysts.
Trophozoites and cysts are scattered individually and in clusters
throughout the neuropil and perivascular spaces. Despite their
similar light microscopic appearance, the two organisms can be
tentatively differentiated by the presence of multiple nucleoli
in B. mandrillaris trophozoites, as opposed to the single nucleolus
of Acanthamoeba spp. trophozoites.
-
- Contributor: PATHVET Consultation Services, 3015 Roxanne
Avenue, Long Beach, CA 90808
-
- References:
1. Lozano-Alarcon F, Bradley GA, Houser BS, Visvesvara GS. Primary
Amoebic Meningoencephalitis due to Naegleria fowleri in a South
American Tapir. Vet Pathol 34(3):239-243, 1997
2. Kinde H, Visvesvara GS, Barr BC, Nordhausen RW, Chiu PHW.
Amebic Meningoencephalitis caused by Balamuthia mandrillaris
(leptomyxid ameba) in a horse. J Vet Diagn Invest 10(4):378-381,
1998
- 3. Rideout BA, Gardiner CH, Stalis IH, Zuba JR, Hadfield
T, Visvesvara GS. Fatal Infections with Balamuthia mandrillaris
(a Free-Living Ameba) in Gorillas and Other Old World Primates.
Vet Pathol 34(1):15-22, 1997
-
-
- Case II - E98-478-2 (AFIP 2686003)
-
- Signalment: Cheetah (Acinonyx jubatus)
-
- Contributor's Diagnosis and Comments: Idiopathic necrotizing
leukoencephalopathy-leukodystrophy
-
- White matter in the corona radiata, internal capsule, and
centrum semiovale is diffusely widened, pale, and crossed by
numerous prominent branching capillaries lined by plump endothelium.
The sheathed axons are also widely separated and the myelin sheaths
are frequently fragmented with the presence of numerous small
round eosinophilic balls. Dispersed throughout the white matter
are a moderate number of foamy gitter cells that occasionally
contain phagocytosed eosinophilic debris. Scattered pyknotic
nuclear debris and a small number of mineralized spherules are
also present. Numerous reactive astrocytes with large round nuclei
and abundant homogenous eosinophilic cytoplasm, form a spiderweb
of interconnected cell processes. Occasionally, these reactive
astrocytes have vacuolated cytoplasm. The grey matter is unaffected
except for a thin rim adjacent to the white matter. Within this
zone of grey matter are a small number of reactive astrocytes
and scattered eosinophilic balls. Occasional vessels are surrounded
by perivascular cuffs composed primarily of lymphocytes along
with rare hemosiderin laden macrophages.
-
- Demyelinating diseases include 1) dysmyelination (genetic
disorders of myelin formation); 2) demyelination secondary to
neuronal destruction (neuronolytic demyelination or Wallerian
degeneration secondary to viral infection or toxicity); and 3)
primary demyelination (diseases where demyelination is the sole
disease process). The CNS lesions in these cheetahs are characterized
by extensive demyelination with at least some degree of concurrent
axonal degeneration. The etiology is uncertain. Transmissible
spongiform encephalopathy has been described in four cheetahs
that were born in captivity in Great Britain. Histopathology
reported in those cases included widespread axonal degeneration
and demyelination of all spinal cord tracts that extended up
the pyramidal tracts in the medulla and as far cranially as the
internal capsule. However, varying degrees of spongiosis was
also seen in grey matter and a few vacuoles were observed within
the perikarya of some neurons.
-
- Through July 1, 1999, a total of 30 confirmed cases of leukoencephalopathy
have been documented in cheetahs. Clinically, affected cheetahs
have evidence of progressive loss of vision, have difficulty
prehending food, and are uncoordinated. All cases have marked
reactive astrocytosis predominantly in the cerebral cortical
white matter with degeneration and necrosis. Lesions seem to
begin in the corona radiata as a reactive astrocytosis that evolves
into demyelination, axonal loss, leukoencephalomalacia, and cavitation
in the oldest lesions. The lesions have remarkable bilateral
symmetry with Wallerian degeneration in descending proprioceptive
pathways, crus cerebri, longitudinal fibers of the pons, the
pyramids, the decussation, and the lateral corticospinal tracts.
-
- Reportedly, the problem first appeared in March, 1997, affects
only older cheetahs, is not familial, has emerged in multiple
facilities in the United States, and has been confirmed in a
single case in England. The same signs and lesions have been
observed in two Florida panthers necropsied in 1994 and 1997.
Both of these animals were at least 8 years old (A. De lahunta,
personal communication). Antemortem diagnosis can be made by
either CT or MR imaging but MRI is the most reliable procedure.
Attempts to identify a possible viral etiology, mycotoxin, Vitamin
B deficiency, or reactions to vaccines or medications are ongoing
(Dr. Linda Munson, UC, Davis).
-
- AFIP Diagnosis: Brain: Leukoencephalopathy characterized
by necrosis, gemistocytic astrocytosis, numerous gitter cells,
mineralization and lymphoplasmacytic inflammation, cheetah (Acinonyx
jubatus), feline.
-
- Conference Notes: Several diseases that are unusual
in most mammals commonly affect captive cheetahs. For example,
gastritis associated with Helicobacter-like organisms, veno-occlusive
disease and glomerulosclerosis have been found to be prevalent
in captive cheetahs in facilities in the United States and the
Republic of South Africa.
-
- Cheetahs are remarkable in their lack of genetic diversity.
It has been hypothesized that a severe population crash might
explain the genetic uniformity of the species. Calculations based
on diversity of mitochondrial DNA and hypervariable minisatellite
loci suggest that the population bottleneck occurred about 10,000
years ago, near the end of the last ice age, in the late Pleistocene.
At that time, extinction of a number of large vertebrates occurred
on several continents. The cheetah's lack of genetic diversity
may play a role in its susceptibility to unusual diseases.
-
- Contributor: College of Veterinary Medicine, Cornell
University, Ithaca, NY 14853-6401
-
- References:
- 1. Allen IV: Demyelinating diseases. In: Greenfield's Neuropathology,
eds. Adams JH, Corselleis JAN, Duchen LW, 4th ed., John Wiley
& Sons, pp. 338-384. New York, NY, 1984
- 2. Baron T, Belli P, Madec JY, Moutou F, Vitaud C, Savey
M: Spongiform encephalopathy in an imported cheetah in France.
Vet Record 141(11):270-271, 1997
- 3. Kirkwood JK, Cunningham AA: Epidemiological observations
on spongiform encephalopathies in captive wild animals in the
British Isles. Vet Record 135(13):296-303, 1994
- 4. Munson L, de Lahunta A, Citino S, Radcliffe R, Neiffer
D, Montali R, Stalis I: Leukoencepahlopathy in cheetahs. Am Assoc
Zoo Vet (abstract), 1999
5. Munson L, Nesbit JW, Meltzer DG, Colly LP, Bolton L, Kriek
NP: Diseases of captive cheetahs (Acinonyx jubatus jubatus) in
South Africa: a 20-year retrospective survey. J Zoo Wildl Med
30(3):342-347, 1999
- 6. Menotti-Raymond M, O'Brien SJ: Dating the genetic bottleneck
of the African cheetah. Proc Natl Acad Sci USA 90(8):3172-3176,
1993
- 7. Peet RL, Curran JM: Spongiform encephalopathy in an imported
cheetah (Acinonyx jubatus). Aust Vet J 69(7):171, 1992
-
-
- Case III- P-081-96 (AFIP 2683470)
-
- Signalment: Three-year-old male rock hyrax (Procavia
capensis)
-
- History: 5 months prior to death, the animal was noted
to have some sneezing, coughing and slight weight loss. The animal's
condition stabilized and the cough became intermittent. Immediately
prior to death, the respiratory signs progressed. A left pulmonary
lobectomy was done during an exploratory thoracotomy. The animal
was electively euthanized the following day due to poor prognosis
based on the results of histopathology.
-
- Gross Pathology: At necropsy, the right lung contained
multifocal to coalescing firm white glistening nodules affecting
up to 60% of the pulmonary parenchyma. The left lung was removed
during surgery 1 day prior to necropsy and had a similar appearance.
-
- Laboratory Results: Cultures are positive for Mycobacterium
bovis.
-
- Histology: Section of lung with severe alterations
of normal architecture. Few remnants of tissue identifying structures
remain. The majority of the sections are effaced by variably
sized 15 to 100 mm diameter nodules. The nodules are centrally
composed of large numbers of whirling epithelioid cells with
oval nuclei and moderate amounts of eosinophilic granular to
vacuolated cytoplasm. Surrounding the nodules are low to moderate
numbers of lymphocytes admixed with plasma cells, and spindle
cells with moderate amounts of eosinophilic fibrillar cytoplasm
(fibrous connective tissue). Multifocal bronchial lumens contains
low numbers of macrophages admixed with lymphocytes and red blood
cells. In one section multifocal bronchi are filled with massive
numbers of mature and degenerate neutrophils admixed with fewer
lymphocytes, plasma cells, macrophages, cellular debris and mucus.
In less severely affected areas, alveolar lumens are filled with
varying combinations of neutrophils, macrophages and large numbers
of red blood cells admixed with homogeneous eosinophilic proteinaceous
material (edema). Remaining pulmonary vessels are filled with
moderate numbers of red blood cells (congestion).
Contributor's Diagnoses and Comments:
- 1. Bronchopneumonia, diffuse, granulomatous, massive with
intralesional acid fast bacilli.
2. Tracheitis, diffuse, granulomatous, severe.
-
- This hyrax died from a severe granulomatous pneumonia and
disseminated granulomas caused by acid fast bacilli. Cultures
of lung tissue isolated Mycobacterium bovis. Acid fast positive
bacilli were found in trachea, lung, thyroid and spleen. In most
sections concentrations of bacilli were low with the exception
of the trachea, where bacilli were more numerous.
-
- AFIP Diagnosis: Lung: Granulomas, epithelioid, coalescing,
rock hyrax (Procavia capensis), cavid.
-
- Conference notes: Mycobacterium spp. are nonmotile,
nonspore forming, pleomorphic bacilli, they are weakly Gram positive
and acid-fast positive. Three species of Mycobacterium are considered
tubercle bacilli, M. tuberculosis, M. bovis, and M. avium. Though
there is some species predilection with each, all three can infect
a wide range of species, and especially immunocompromised animals.
-
- Due to the presence of several compounds in their cell walls,
mycobacteria are able to escape killing by phagocytic cells and
induce delayed hypersensitivity. Cord factor is a surface glycolipid
found in pathogenic mycobacteria; it stimulates granuloma formation.
Sulfatides are sulfur-containing glycolipids that prevent fusion
of phagosomes of macrophages with lysosomes. Another cell wall
constituent, lipoarabinomannan (LAM), is a heteropolysaccharide
that inhibits macrophage activation by interferon-gamma and induces
macrophages to secrete TNF-a and IL-10, which suppresses mycobacteria-induced
T-cell proliferation.
-
- The mycobacteria are initially able to replicate in naive
macrophages. After a few weeks, however, T cell mediated immunity
develops (delayed hypersensitivity). CD4+ helper T cells activate
macrophages by secreting interferon-gamma, enabling the macrophages
to kill the bacilli via the release of reactive nitrogen intermediates.
CD8+ suppressor T cells kill macrophages that harbor mycobacteria,
causing caseous necrosis; the mycobacteria cannot grow in the
acidic, extracellular environment of the caseous core of a granuloma.
The classic granuloma of tuberculosis is the result of this process.
-
- Chronic infection frequently follows, with a balance between
bacterial replication and destruction. Stress of any kind can
tip the scale in the direction of the mycobacteria and produce
fulminant infection.
-
- Contributor: Department of Pathology, Wildlife Health
Center / WCS, 185th St. and Southern Blvd. Bronx, New York, 10460
-
- References:
- 1. Hines ME, Kreeger JM, Herron AJ: Mycobacterial Infections
of Animals: Pathology and Pathogenesis, Lab An Sci 45(4):334-347,
1995
- 2. Jackson R, Cooke MM, Coleman JD, Morris RS, de Lisle GW,
Yates GF: Naturally occurring tuberculosis caused by Mycobacterium
bovis in brushtail possums (Trichosurus vulpecula): III. Routes
of transmission and excretion. NZ Vet J 43:322-327, 1995
- 3. Krebs JR, Anderson RM, Clutton-Brock T, Donnelly CA, frost
S, Morrison WI, Woodroffe R, Young D: Badgers and bovine TB:
Conflicts between conservation and health. Science 279:817-818,
1998
- 4. Miller J, Jenny A, Rhyan J, Saari D, Suarez D: Detection
of Mycobacterium bovis in formalin-fixed, paraffin-embedded tissues
of cattle and elk by PCR amplification of an IS6110 sequence
specific for Mycobacterium tuberculosis complex organisms. J
Vet Diag Lab Inv 9:244-249, 1997
- 5. Rhyan J, Saari D: A conparative study of the histopathologic
features of bovine tuberculosis in cattle, fallow deer (Dama
dama), Sika deer (Cervus nippon), red deer and elk (Ce-rvus elaphus).
Vet Pathol 32:215-220, 1995
- 6. Samuelson J: Infectious diseases. In: Robbins Pathologic
Basis of Disease, eds. Cotran RS, Kumar V, Collins T, 6th ed.,
pp. 349-352. WB Saunders, Philadelphia, 1999
-
-
- Case IV - X6664 (AFIP 2695443)
-
- Signalment: 8-year-old, female, captive red panda
(Ailurus fulgens)
-
- History: Traumatic event resulted in fracture of ulna
and muscular damage around axilla. The red panda died 3 days
later.
-
- Contributor's Diagnosis and Comments: Stomach: Erosions
and ulcers, acute, multifocal, with fibrinous thrombi
- Etiology: Associated with physiological stress
-
- Histologically, there is multifocal degeneration and acute
coagulative necrosis and loss of gastric mucous neck cells, chief
cells, and parietal cells. There are superficial erosions of
the gastric mucosa that sometimes extend deep to the vicinity
of the muscularis mucosa. Multifocally, there is brown, granular
to globular pigment along the base and around few distended veins
in some of the eroded areas. At the base of the mucosa, there
is venous distention with margination of neutrophils and, in
some areas, infiltration of the eroded mucosa by few neutrophils.
Few ectatic veins within the lamina propria contain intravascular
fibrin thrombi. In few sections of stomach, there is ulceration
of the mucosa that is characterized by extension of the mucosal
necrosis to the muscularis mucosa.
-
- Generally, erosions and ulcers of this type are usually associated
with physiological stress. The "stress ulcers" can
occur multifocally throughout the gastric and sometimes duodenal
mucosa. In humans, they have been associated with shock, sepsis,
burns, trauma, and increased cranial pressure. The pathogenesis
of stress ulcers is still not completely understood but possible
mechanisms are decreased blood flow to the mucosa (ischemic necrosis),
disruption of the gastric mucous layer, decreased bicarbonate
buffer, increased acid secretion, and direct damage to the gastric
mucosal epithelium.
-
- In wild and captive nondomestic animals, stress erosions
and ulcers are not uncommon. Spontaneous acute gastric erosions
and ulcers due to physiological stress have been previously reported
in captive vervet monkeys (Cercopithecus aethiops). In one study,
stress ulcers occurred in approximately 1/3 of necropsied monkeys
and were associated with individual housing conditions. Gastric
ulcers have been reported in stranded marine mammals such as
the sperm whale and were noted in sea lion pups affected by ecological
disruptions associated with El Nino. Llamas hospitalized for
extended periods of time have been known to develop third compartment
ulcers. In the case of this red panda, the stress associated
with the traumatic injuries most likely caused the gastric erosions
and ulcers.
-
- AFIP Diagnosis: Stomach, mucosa: Erosions and necrosis,
multifocal, acute, with fibrin thrombi, red panda (Ailurus fulgens),
procyonid.
-
- Conference Notes: Gastric erosions and ulcers are
common in most domestic and exotic species and have been associated
with many etiologies, including stress, septicemia, uremia, disseminated
intravascular coagulation, glucocorticoid usage, and administration
of non-steroidal anti-inflammatory drugs. In dogs, gastric ulcers
are occasionally associated with gastrin-secreting pancreatic
tumors and mast cell tumors. Ulcers in the pars esophagea region
of pigs are associated with the practice of feeding finely ground
feed.
Gastric erosions are characterized by loss of the superficial
epithelium that produces a defect that does not cross the muscularis
mucosae. Frequently there is an associated acute inflammatory
infiltrate and extrusion of a fibrin-containing purulent exudate
into the lumen. Gastric ulcer is defined as a breach of the mucosa
that extends through the muscularis mucosa into the submucosa
or deeper.
-
- Contributor: Department of Pathology, National Zoological
Park, Washington, DC 20008
-
- References:
- 1. Crawford JM: The Gastrointestinal Tract. In: Pathologic
Basis of Disease, eds. Cotran RS, Kumar V, Collins T, 6th edition,
WB Saunders Company, Philadelphia, PA, 1999
- 2. Jauniaux T, Brosens L, Jacquinet E, Lambrights D, Addink
M, Smeenk C, and Coignoul F: Postmortem investigations on winter
stranded sperm whales from the coasts of Belgium and The Netherlands.
J Wildl Dis 34: 99-109, 1998
- 3. Mbaruk AS, Tarara RP, Else JG, Sayer PD: Spontaneous acute
gastric mucosal erosions and ulcerations in vervet monkeys. J
Zoo Wildl Med 26: 67-71, 1995
- 4. Spraker TR, Gulland F, DeLong R: The impact of El Nino
on marine mammals. Proc Am Assoc Zoo Vet: 160-161, 1998
- 5. Smith BB, Pearson EG, Timm KI: Third compartment ulcers
in the llama. Vet Clin North Am Food Anim Pract 10: 319-30, 1994
- 6. Tarara MA, Tarara RP, Suleman MA: Stress-induced gastric
ulcers in vervet monkeys: The influence of life history factors.
J Zoo Wildl Med 26: 72-75, 1995
-
- J Scot Estep, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: estep@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
- Return to WSC Case Menu
