Results
AFIP Wednesday Slide Conference - No. 23
March 10, 1999
- Conference Moderator:
MAJ (P) Mark Mense
Walter Reed Army Institute of Research
Division of Pathology
Washington, D.C. 20307-5100
-
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
-
Case I - 12A (AFIP 2658211)
- Signalment: 17-year-old, male, Japanese macaque (Macaca
fuscata).
-
- History: This monkey was from a large, free-ranging
colony located in south Texas (Dilly, Texas). The animal was
incontinent, paretic, and paralyzed in the hind limbs. Three
of four limbs were edematous. The animal was euthanized for humane
reasons.
-
- Gross Pathology: Externally, there was massive subcutaneous
edema over the ventral body, legs, and scrotum. Internally, there
were marked hydropericardium and ascites. The lungs were multifocally
abscessed and infarcted. There were tan to yellow, 2 mm, slightly
raised, miliary foci in the lungs, liver, spleen, and kidneys.
Gross diagnoses were congestive heart failure, ascites, hydropericardium,
and multiple organ abscesses.
-
- Laboratory Results: None.
-
- Contributor's Diagnosis and Comments: Heart: Pericarditis
and epicarditis, pyogranulomatous, diffuse, severe, with fungal
spherules, Japanese macaque (Macaca fuscata), primate. Etiology
consistent with Coccidioides immitis.
-
- In the submitted section of heart, the pericardium is greatly
expanded by pyogranulomatous inflammation and necrotic debris
that extend through the epicardium. The inflammation is composed
of degenerate neutrophils, macrophages, and multinucleated foreign
body giant cells, with fewer lymphocytes and plasma cells. Multifocally,
these form small, well to poorly organized, discrete granulomas.
Within giant cells and free in the necrotic milieu, there are
15 to 45mm diameter spherules with a 4 to 5mm thick, birefringent
wall, containing flocculent basophilic granular material (immature
stages) or 5 to 7mm diameter endospores (mature endosporulating
stages). Other spherules are fragmented and degenerate. Similar
yeast organisms were found within pyogranulomatous foci in the
lung, spleen, pancreas, lymph nodes, and liver.
-
- Coccidioides immitis is a dimorphic fungus known to infect
humans, wild and domestic animals, nonhuman primates, rodents,
and exotic animals in the southwestern United States, northern
Mexico, and in endemic areas of Central and South America. Inhalation
of fungal spores is the only proven mode of natural infection,
although direct transmission has been implicated. Fatal coccidioidomycosis
has been diagnosed in several nonhuman primate species including
the rhesus, bonnet macaque, Japanese macaque, chimpanzee, sooty
mangabey, gorilla, and baboons.
-
- Endosporulating mature spherules contain numerous uninucleate
endospores and can measure more than 30mm in diameter. Immature
spherules contain granular material. Differential diagnosis includes
Rhinosporidium seeberi, Chrysosporium parvum var. crescens, Cryptococcus
neoformans, Paracoccidioides brasiliensis, Histoplasma capsulatum,
Emmonsia parva, and Blastomyces dermatitidis, but usually these
can easily be eliminated from diagnostic consideration by histologic
features, anatomic location, and host reaction. Complement fixation,
immunodiffusion, and fluorescent antibody tests diagnostic for
C. immitis are available and may be very useful in difficult
cases.
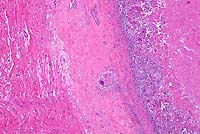 4x
obj.
4x
obj.
- Case 23-1. Heart. The epicardium is markedly thickened
by fibrosis and a superficial exudate composed of caseous necrotic
debris, a dense cellular infiltrate, with multinucleate giant
cells and scattered 50u diameter yeast spherules.
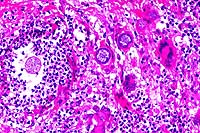 20x
obj.
20x
obj.
- Case 23-1. Epicardial exudate. Immature spherules
have a hyaline eosinophilic capsule and contain granular basophilic
material. Mature spherules contain abundant 2u diameter endospores.
Spherules are surrounded by numerous neutrophils, fewer macrophages,
multinucleate foreign body giant cells, lymphocytes and plasma
cells.
-
- AFIP Diagnoses:
- 1. Heart, epicardium: Granuloma, with mature and immature
fungal spherules, Japanese macaque (Macaca fuscata), nonhuman
primate, etiology consistent with Coccidioides immitis.
2. Heart: Fibrosis, interstitial, multifocal, mild, with multifocal
myofiber atrophy and karyomegaly.
-
- Conference Note: Occurrence of coccidioidomycosis
in animals and humans is typically associated with arid or semi-arid
environments, such as the southwestern United States and parts
of Mexico. In humans, the disease is commonly referred to as
San Joaquin Valley Fever. Animals most commonly infected are
dogs, horses, and feedlot cattle. The infection in cattle is
often subclinical and restricted to the lungs.
-
- Interestingly, a case of pulmonary coccidioidomycosis has
been recently reported in a stranded bottlenose dolphin from
the southern California coast. While coccidioidomycosis has been
previously described in marine mammals such as sea otters and
California sea lions, the infection in the bottlenose dolphin
is unique because it is the first report of coccidioidomycosis
in a purely aquatic, free-ranging marine mammal. Coccidiodes
immitis can be carried long distances by the wind, and can survive
in saline soil and sea water. Bottlenose dolphins venture close
to the California shore, and it was speculated that an offshore
wind may have carried infectious arthrospores to the dolphin
from an endemic area in California.
- Mild myocardial fibrosis and karyomegaly of myocardial fibers
are common findings in aged macaques and are considered incidental
in this case.
-
- Contributor: Southwest Foundation for Biomedical Research,
Air Force Research Laboratory, 2509 Kennedy Circle, Brooks Air
Force Base, Texas 78235.
-
- References:
- 1. Bellini S, Hubbard G, Kaufman L: Spontaneous fatal coccidioidomycosis
in a native-born hybrid baboon (Papio cynocephalus anubis/Papio
cynocephalus cynocephalus). Lab Anim Sci 41:509-511, 1991.
- 2. Johnson HJ, et al.: Disseminated coccidioidomycosis in
a mandrill baboon (Mandrillus sphinx): A case report. J Zoo Wildl
Med 29:208-213, 1998.
- 3. Reidarson TH, Griner LA, Pappagianis D, McBain J: Coccidioidomycosis
in a bottlenose dolphin. J Wildl Dis 34:629-631, 1998.
- 4. Ziemer EL, et al.: Coccidioidomycosis in horses: 15 cases.
J Amer Vet Med Assoc 201:910-916, 1992.
- 5. Samuelson J: Infectious diseases. In: Robbins Pathologic
Basis of Disease, Cotran RS, Kumar V, Collins T, eds., 6th ed.,
page 353, WB Saunders, Philadelphia, PA, 1999.
-
Case II - NADC MVP-2 (AFIP 2638852)
-
- Signalment: Adult, female, white-tailed deer (Odocoileus
virginianus).
-
- History: This deer was from a herd of 700 captive
white-tailed deer on a hunting preserve that was depopulated
due to Mycobacterium bovis infection. All deer in the herd were
in good flesh. No clinical signs had been noted.
-
- Gross Pathology: Medial retropharyngeal lymph nodes
were enlarged to 3 to 4 cm in diameter. On cut surface there
was caseous necrosis and mineralization surrounded by fibrous
connective tissue and more normal lymphoid tissue.
-
- Laboratory Results: Mycobacterium bovis was cultured
from samples of the affected lymph nodes. PCR of formalin-fixed,
paraffin-embedded sections to detect an insertion sequence specific
to M. tuberculosis complex mycobacteria also confirmed the presence
of M. bovis.
-
- Contributor's Diagnosis and Comments: Lymph node:
Granulomatous lymphadenitis, multifocal to coalescing, with caseous
necrosis, mineralization, and peripheral fibrosis.
-
- Mycobacterium bovis, the causative agent of tuberculosis
in cattle, is also the cause of tuberculosis in Cervidae. Captive
as well as free-ranging Cervidae have been diagnosed with M.
bovis infection. A recent outbreak of M. bovis infection in wild
white-tailed deer in Michigan represents the first known wild
animal reservoir of M. bovis in North America. White-tailed deer
come into close contact with cattle and have been seen to share
feeding and watering sites. Other wild animal reservoirs for
M. bovis include the brushtail possum (Trichosurus vulpecula)
in New Zealand and the badger (Meles meles) in the United Kingdom.
Wildlife reservoirs of M. bovis represent a serious threat to
efforts to eradicate tuberculosis from domestic livestock. Possums
and badgers have been documented as a source of infection in
domestic cattle herds grazing pastures where infected possums
and badgers reside.
Tuberculosis in some species of Cervidae have been found to have
lesions morphologically distinct from typical bovine lesions.
Elk and red deer (Cervus elaphus) have peripheral mineralization
of granulomas rather than the central mineralization often seen
in cattle. Lesions in elk and red deer often have more neutrophils
and fewer giant cells than lesions seen in cattle. Fallow deer
(Dama dama) generally have more giant cells than bovine lesions,
but are otherwise indistinguishable from bovine lesions. Sika
deer (Cervus nippon) have more giant cells which are larger with
more nuclei than giant cells seen in bovine lesions. Lesions
in white-tailed deer have been described as typical of lesions
seen in cattle. Medial retropharyngeal lymph nodes have been
found to be the most common site to contain lesions in white-tailed
deer.
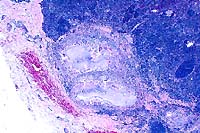 4x
obj.
4x
obj.
- Case 23-2. Lymph node. There is a caseating granuloma
displacing cortical lymphocytes which contains foci of mineralization
and giant cells.
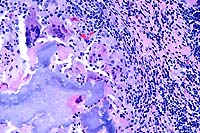 20x
obj.
20x
obj.
- Case 23-2. Lymph node. Partly mineralized caseous
necrotic debris is surrounded by multiple foreign body giant
cells, epithelioid macrophages, lymphocytes, and plasma cells
(tubercle).
-
- AFIP Diagnosis: Lymph node: Granuloma(s), caseo-calcareous,
white-tailed deer (Odocoileus virginianus), cervid.
-
- Conference Note: There is marked variability of the
examined sections. Some slides contain single to multiple, small,
discrete granulomas within clearly identifiable sections of lymph
node. In other slides, the lymph node is enlarged and the parenchyma
is almost entirely replaced by a single, large, caseo-calcareous
granuloma; remnant lymphoid elements are present at the periphery
of the lymph node. Staining by Fite's method for acid-fast bacteria
performed at the AFIP demonstrated rare, acid-fast bacilli within
macrophages.
-
- Mycobacteria are aerobic, nonmotile, non-spore forming bacilli
characterized by a waxy coat that retains red dye when subjected
to acid in acid-fast stains. The pathogenicity of M. tuberculosis
infection has been attributed to several components in the bacterial
cell wall that allow the organism to escape killing by macrophages
and induce delayed type hypersensitivity. Virulent strains of
M. tuberculosis possess cord factor, a glycolipid on the surface
of the bacterium. In mice, injection of purified cord factor
induces granuloma formation. Lipoarabinomannan, a polysaccharide
similar in structure to that of endotoxin in Gram-negative bacteria,
inhibits macrophage activation by interferon-g, induces macrophage
secretion of TNF-a, causing fever and weight loss, and causes
secretion of IL-10, which suppresses mycobacteria induced T-cell
proliferation. Complement, activated on the surface of bacilli,
serves to opsonize the organisms and facilitate their uptake
into macrophages. In addition to the virulence factors associated
with the cell wall, mycobacteria reside in phagosomes that fail
to become acidified. Lack of acidification of lysosomes has been
associated with urease secreted by mycobacteria, and with phagocytosis
of bacteria via complement or mannose binding receptors rather
than Fc receptors.
-
- Contributor: National Animal Disease Center, 2300
Dayton Road, Ames, Iowa 50010.
-
- References:
- 1. Rhyan JC, Saari DA: A comparative study of the histopathologic
features of bovine tuberculosis in cattle, fallow deer (Dama
dama), Sika deer (Cervus nippon), and Red deer (Cervus elaphus).
Vet Pathol 32:215-220, 1995.
- 2. Schmitt SM, Fitzegerald SD, Cooley TM, et al.: Bovine
tuberculosis in free-ranging white-tailed deer from Michigan.
J Wild Dis 17:749-758, 1997.
- 3. Miller J, Jenny A, Rhyan J, et al.: Detection of Mycobacterium
bovis in formalin-fixed, paraffin-embedded tissues of cattle
and elk by PCR amplification of an IS6110 sequence specific for
Mycobacterium tuberculosis complex organisms. J Vet Diag Lab
Inv 9:244-249, 1997.
- 4. Krebs JR, Anderson RM, Clutton-Brock T, et al.: Badgers
and bovine TB: Conflicts between conservation and health. Science
279:817-818, 1998.
- 5. Morris RS, Pfeiffer DU: Directions and issues in bovine
tuberculosis epidemiology and control in New Zealand. NZ Vet
J 43:256-265, 1995.
- 6. Jackson R, Cooke MM, Coleman JD, et al.: Naturally occurring
tuberculosis caused by Mycobacterium bovis in brushtail possums
(Trichosurus vulpecula): III. Routes of transmission and excretion.
NZ Vet J 43:322-327, 1995.
- 7. Samuelson J: Infectious diseases. In: Robbins Pathologic
Basis of Disease, Cotran RS, Kumar V, Collins T, eds., 6th ed.,
pp. 349-352, WB Saunders, Philadelphia, 1999.
-
Case III - 7-78-96 (AFIP 2657523)
- Signalment: One-month-old greater rhea (Rhea americana).
-
- History: Three juvenile (one to three-month-old) rheas
from a flock of 200 birds died with leg weakness and lethargy.
-
- Gross Pathology: The necropsy was performed by the
submitting veterinarian. Within the liver there were disseminated,
pale, friable foci up to 3 mm in diameter. The liver was enlarged,
pale, and firm.
-
- Laboratory Results:
- 1. Liver selenium: 3.64 mg/g dry weight.
2. Liver Vit E: 28.92 mg/g dry weight.
3. Liver: Mg 184 ppm, Cu 3.67 ppm, Zn 154 ppm, Mn 1.99 ppm, Cd
<0.10 ppm, and Mo 0.523 ppm.
-
- Contributor's Diagnosis and Comments:
- 1. Liver: Granulomata, heterophilic, multiple.
- 2. Liver: Amyloidosis, multifocal and coalescing, marked.
-
- A Gram-negative, curved rod was isolated from the liver.
The isolate was identified as Campylobacter coli, an enteric
pathogen in ratites. Systemic infection with associated hepatic
lesions and encephalitis attributed to Campylobacter jejuni has
been previously documented in juvenile ostriches. Lesions include
focal hepatic necrosis, ascites, hydropericardium, and swollen
kidneys. The liver copper level was below normal range in this
case, and may have been contributory. Hepatic amyloid deposition
is commonly encountered in birds with chronic and active inflammatory
processes.
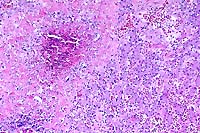 10x
obj.
10x
obj.
- Case 23-3. Liver. A granuloma composed of necrotic
debris surrounded by waxy eosinophilic material (amyloid) and
degenerate inflammatory cells replaces hepatic parenchyma.
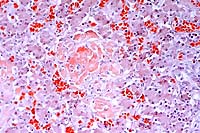 Congo
red, 20x obj.
Congo
red, 20x obj.
- Case 23-3. Liver. Hepatic plates are separated by
amorphous deposits of red staining material (amyloid) and low
numbers of lymphocytes.
-
- AFIP Diagnosis: Liver: Granulomas, heterophilic, with
multifocally extensive amyloidosis, greater rhea (Rhea americana),
avian.
-
- Conference Note: Conference participants considered
a variety of bacteria as potential causes of the hepatic lesions
observed in this greater rhea. Infection with Mycobacterium avium
was the primary consideration in the differential diagnosis of
most attendees. Escherichia coli, Salmonella sp., and Campylobacter
sp. were also mentioned. Ziehl-Neelsen and Fite's acid-fast stains
performed at the AFIP did not demonstrate acid-fast bacteria.
Additionally, tissue Gram stains, the Warthin-Starry method and
Steiner's method did not demonstrate bacteria. A Congo red stain
confirmed the presence of amyloid.
-
- A variety of pathogenic bacteria may cause similar histologic
lesions in the livers of birds. While conference participants
agreed that the hepatic lesions and culture results in this rhea
are consistent with campylobacteriosis, some were reluctant to
definitively attribute the changes to Campylobacter sp. without
other evidence of its presence within the liver. Bacterial culture
results are most reliable when interpreted in context with histopathologic
findings and observation of bacteria within lesions. Techniques
such as in situ hybridization and immunohistochemistry are often
very useful in demonstrating infectious agents in lesions that
contain few microorganisms.
Contributor: Montana Veterinary Diagnostic Laboratory,
PO Box 997, Bozeman, Montana 59771.
-
- References:
- 1. Jensen J, Johnson, JH, Weiner ST: Husbandry and medical
management of ostriches, emus and rheas. Wildlife and Exotic
Animal TeleConsultants, 1992.
- 2. Perelman JB: Campylobacteriosis. In: Proceedings of Third
Annual Ostrich Conference: Ostrich Medicine and Surgery for Veterinarians,
College of Veterinary Medicine, Texas A&M University, 1991.
- 3. Post K, Ayers JR, Gilmore WC, Raleigh RH: Campylobacter
jejuni isolated from ratites. J Vet Diagn Invest 4:345-437, 1992.
- 4. Boukraa L, Messier S, Robinson Y: Isolation of Campylobacter
from livers of broiler chickens with and without necrotic hepatitis
lesions. Avian Dis 35:714-717, 1991.
- 5. Oyarzabal OA, Conner DE, Hoer FJ: Incidence of campylobacters
in the intestine of avian species in Alabama. Avian Dis 39:147-151,
1995.
Case IV - CID (AFIP 2658212)
- Signalment: Adult feral pig.
-
- History: Tissue was collected from one of several
pigs slaughtered at a local meat processing plant. The pigs were
heavily infested with various metazoan parasites, including lungworms,
roundworms, tapeworms, and acanthocephalids.
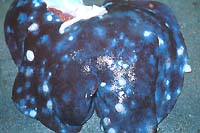
- Case 23-4. Liver. Hepatic parenchyma is discolored
by numerous 2-3cm white foci. Occasionally thin walled white
cysts elevate the hepatic serosa.

- Case 23-4. Kidneys, perirenal fat, ureters. Several
1-2cm cysts are scattered throughout the perirenal fat. A ruptured
cyst contains a creamy brown exudate.
-
- Gross Pathology: Multifocal and coalescing, firm,
nodular areas were observed markedly thickening the loose fibrous
connective tissue surrounding the renal hilus and ureters. When
incised, the fibrotic nodules were abscessed and contained encysted
adult nematodes 2 to 4 cm in length. Multifocal, white, irregularly
round to linear areas of fibrosis were present in the subcapsular
and interstitial tissue of the liver.
-
- Contributor's Diagnosis and Comments: Perirenal and
periureteral connective tissue: Granulomas, eosinophilic, multifocal,
with intralesional adult nematodes and eggs, etiology consistent
with Stephanurus dentatus.
-
- Infestation with Stephanurus dentatus, the kidney worm of
swine, is a common problem of feral swine in most tropical and
subtropical climates. Clinical findings in affected animals vary
with the magnitude of infection and location of migrating larvae,
ranging from stunted growth to emaciation, ascites, rear limb
lameness, and posterior paresis. Pigs are initially infected
with the 3rd stage larvae via ingestion, skin penetration, or
prenatally. Ingested larvae migrate through the portal circulation
or lymphatics to the liver, while those acquired cutaneously
pass to the lungs prior to reaching the liver via the systemic
circulation. Grossly, S. dentatus migration through the liver
can often be distinguished from that of Ascaris suum by the severe
inflammation and tract-like, irregular pattern of fibrosis produced
by the former. Severe hepatitis leading to cirrhosis and ascites
can occur. Larvae in the liver are destroyed, encapsulated or
eventually break through the hepatic capsule, migrating to the
preferred perirenal and mesenteric tissue sites. Other lesions
ascribed to aberrant larval migration of S. dentatus in pigs
include portal phlebitis, pancreatitis, splenitis, lymphadenitis,
myositis, and myelitis leading to paralysis.
-
- The encysted perirenal larvae develop into adults. Perirenal
cysts typically contain a pair of worms surrounded by inflammatory
cells and a dense fibrous capsule. The cysts communicate with
the ureter allowing large numbers of eggs to pass freely in the
urine of the host. The eggs develop into the infective L3 larval
stage approximately four days later, completing the life cycle.
Larvae can survive in the appropriate environment for up to five
months. Once infected, up to nine months may be required to establish
a patent infection in the host animal.
-
- Features which help to identify the adult worms in these
sections as true strongyles include the platymyarian, meromyarian
musculature, prominent lateral chords, a pseudocoelom, and a
relatively large intestine composed of few, multinucleated cells
which display a thick eosinophilic microvillar border ("strongyle
gut"). Thin-walled, morulated eggs can be seen in the reproductive
tract and free in surrounding tissue in most sections.
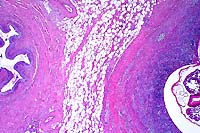 2x
obj.
2x
obj.
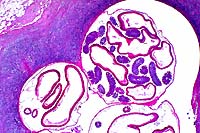
- Case 23-4. Ureter, perirenal fat. There is a large
granuloma within the periureteral fat which contains a cross
section of a nematode parasite.
 2x
obj.
2x
obj.
- Case 23-4. Parasitic granuloma. Three cross-sections
of a nematode parasite contain profiles of ovaries and multiple
cross-sections of nematode digestive tract.
-
- AFIP Diagnosis: Fibrous and adipose tissue, periureteral
(per contributor): Eosinophilic granulomas, multiple, with globule
leukocytes, and adult nematodes and eggs, feral pig, porcine,
etiology consistent with Stephanurus dentatus.
-
- Conference Note: The swine kidney worm measures 20-40
mm in length and is found principally in perirenal fat and adjacent
tissues. The nematode is especially common in the southern United
States. Earthworms can serve as transport hosts. As described
by the contributor, extensive migration by the parasite may produce
widespread tissue damage and a variety of clinical signs. Stephanurus
dentatus has been found in various organs and tissues including
the kidneys, lumbar muscles, heart, lungs, pleural cavity, spleen,
and spinal canal. Differential diagnosis considered by conference
participants included Dioctophyma renale, the giant kidney worm
of several animal species including swine, and aberrant migration
of Ascaris suum.
-
- Contributor: Wilford Hall Medical Center, 59th MDW/MSR,
1255 Wilford Hall Loop, Lackland Air Force Base, Texas 78236.
-
- References:
- 1. Blood DC, Henderson JA, Radostits OM: Diseases caused
by helminth parasites. In: Veterinary Medicine, 5th ed., pp.
780-782, Lea & Febiger, Philadelphia, 1979.
- 2. Soulsby EJL: Class: Nematoda. In: Helminths, Arthropods,
and Protozoa of Domesticated Animals, 7th ed., pp. 193-195, Lea
& Febiger, Philadelphia, 1982.
- 3. Corwin, DiMarco, McDowell, Pratt: Internal parasites.
In: Diseases of Swine, Leman AD, ed., 6th ed., pp. 658-659, Iowa
State Univ. Press, Ames, IA, 1986.
- 4. Maxie GM: The urinary system. In: Pathology of Domestic
Animals, Jubb KVF, Kennedy PC, Palmer N, eds., 4th ed., vol.
2, pp. 516-517, Academic Press, 1993.
- 5. Jones TC, RD Hunt, NW King: Diseases caused by parasitic
helminths and arthropods. In: Veterinary Pathology, 6th edition,
page 648, Williams and Wilkins, Baltimore, MD, 1997.
-
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
-
- Return to WSC Case Menu
 4x
obj.
4x
obj.
 20x
obj.
20x
obj.
 4x
obj.
4x
obj.
 20x
obj.
20x
obj.
 4x
obj.
4x
obj.
 20x
obj.
20x
obj.
 10x
obj.
10x
obj.
 Congo
red, 20x obj.
Congo
red, 20x obj.


 2x
obj.
2x
obj.
 2x
obj.
2x
obj.