Results
AFIP Wednesday Slide Conference - No. 21
February 24, 1999
- Conference Moderator:
COL Michael J. Langford
Division of Pathology
Walter Reed Army Institute of Research
Washington, DC 20307-5100
-
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
-
Case I - H98-0948 (AFIP 2641252)
- Signalment: Ten-month-old, Hereford steer.
-
- History: Steers in a commercial feedlot were fed a
diet to which sodium molybdate had been accidentally added (instead
of sodium bicarbonate) at a rate of 1.9% of the total ration.
Affected animals were inappetent and weak. Of the 800 steers
in the consignment, 90 died.
-
- Gross Pathology: There were widespread subcutaneous
petechial hemorrhages. The liver was pale tan, with irregular,
focal, 3-5 mm diameter hemorrhages on both the capsular and cut
surface. The kidneys were swollen and pale.
-
- Laboratory Results: In a euthanized animal that had
access to the contaminated feed for up to 6 days, the plasma
molybdenum concentrations were 13,000 mg/L (430 times the normal
level). Kidney molybdenum concentrations were 21mg/kg (15 times
the normal level), and liver molybdenum concentrations were 12
mg/kg (12 times the normal levels). Fungal cultures of feed samples
were negative.
-
- Contributor's Diagnoses and Comments:
- 1. Liver: Necrosis, acute, diffuse, severe with mild neutrophilic
cholangitis and pericholangitis.
2. Kidney: Tubular necrosis, acute, moderate.
Etiology: Molybdenum toxicity.
-
- The contaminated diet in this outbreak contained 7400 mg
of molybdenum per kilogram of feed. The rapid rise in plasma
molybdenum concentrations suggest that in cattle, as in humans,
molybdenum absorption is passive and non-saturable. Tissue molybdenum
levels had returned to normal concentrations within 70 days of
the contaminated feed being withdrawn. Previous reports of molybdenosis
in cattle have involved dietary concentrations of molybdenum
between 20 and 200 mg/kg, and symptoms were consistent with a
molybdenum-induced copper deficiency.
-
- The gross and histopathological changes seen were suggestive
of a fungal or plant toxin. The hepatic lesions of periacinar
to massive necrosis accompanied by hemorrhage were suggestive
of toxicity by blue-green algae (Nodularia spumigena or Microcystis
aeruginosa), zamia palm (Macrozamia reidlei) or by Myoporum insulare
("boobialla"), a plant known to grow in the area. Examination
of water from troughs, dams and holding tanks showed no evidence
of blue-green algae, and cattle had no access to trees or shrubs.
Hepato-renal lesions have been described with toxicity associated
with the ingestion of Lantana sp., or with plants containing
polyphenols or tannins, such as yellow wood (Terminalia oblongata),
black wattle (Acacia salicina) and supplejack (Ventilago viminalis).
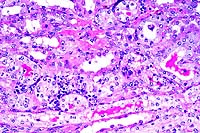 20x
obj.
20x
obj.
- Case 21-1. Kidney. There is pyknosis and karyorrhexis
of tubular epithelial cells (necrosis). Other tubular epithelial
cells are hyperchromatic with large open nuclei (suggests regeneration).
There is a mild interstitial lymphocytic infiltrate.
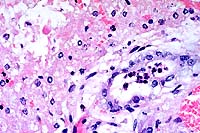 40x
obj.
40x
obj.
- Case 21-1. Liver. Bile ducts contain neutrophils and
are surrounded by edema. There is degeneration and necrosis of
adjacent hepatocytes.
-
- AFIP Diagnoses:
- 1. Kidney: Necrosis and regeneration, tubular, diffuse, with
granular and hyaline casts, Hereford, bovine.
2. Kidney: Nephritis, interstitial, lymphoplasmacytic, multifocal,
mild.
3. Liver: Necrosis, disseminated.
4. Liver: Cholangiohepatitis, neutrophilic, diffuse, mild.
-
- Conference Note: Participants identified extensive,
sometimes submassive, hepatic necrosis and neutrophilic portal
inflammation that multifocally filled bile ducts and disrupted
the limiting plate. Viable hepatocytes were present among individually
dissociated and necrotic hepatocytes. In addition to necrosis
of the tubular epithelium in the kidney, tubular regeneration
and casts were observed.
-
- Molybdenum is an essential trace element in plants, humans,
and ruminants. The metal is necessary for plants to fix nitrogen,
while in animals it serves as a cofactor in the enzymes xanthine
oxidase, aldehyde oxidase, and sulfite oxidase. Within the body,
molybdenum is concentrated in the liver, kidneys, bones, pancreas,
adrenal glands, and omentum. Molybdenum elimination occurs primarily
through the renal system, with over 50% of excreted molybdenum
found in the urine; some is excreted through perspiration in
humans.
-
- While the lesions in the steer represent acute molybdenum
toxicosis, the vast majority of reported cases are due to chronic
toxicity primarily associated with ingestion of green pasture
plants grown on soils high in molybdenum, such as muck or shale
soils in Florida, California, and the western United States ("teart"
soils in England). Ruminants are most commonly affected, especially
cattle. Clinical signs and pathological findings reflect disorders
of the integumentary, musculoskeletal, reproductive, hematolymphatic,
and gastrointestinal systems. Clinical signs include emaciation,
lethargy, rough hair coat, malodorous diarrhea, pale mucous membranes
(due to anemia), sterility, and reluctance to move or pain upon
locomotion. Pathological findings include microcytic hypochromic
anemia, enlargement of long bone epiphyses and costochondral
articulations in young animals, rarefaction of bone and fractures
in older animals, and increased developmental anomalies in neonates
born to affected animals. Permanent aspermatogenesis occurs in
bulls, and while reversible ovarian dysfunction may be present
in cows.
-
- Contributor: Agriculture Western Australia, Division
of Veterinary and Biomedical Sciences, Murdoch University, South
Street, Murdoch, Western Australia 6150.
-
- References:
- 1. Swan DA, et al.: Molybdenum poisoning in feedlot cattle.
Aust Vet J 76:345-349, 1998.
- 2. Jones TC, Hunt RD, King NW: Diseases due to extraneous
poisons. In: Veterinary Pathology, 6th ed., page 736, Williams
and Wilkins, Baltimore, MD, 1997.
- 3. Goyer RA: Toxic effect of metals. In: Casarett and Doull's
Toxicology: The Basic Science of Poisons, Klaassen CD, ed., 5th
edition, page 718, McGraw-Hill, New York, 1996.
-
Case II - 97-3319 (AFIP 2648171)
- Signalment: Five-month-old, male, Ile de France, ovine.
-
- History: Several animals out of a group of 200 Ile
de France sheep reared in the Mpumalanga Province (north-eastern
region) of South Africa developed nervous symptoms. Affected
sheep initially displayed an unsteady and stiff gait which progressed
over the course of a few days to total spasticity, convulsions
and lateral recumbency with paddling, followed by death. Sheep
of both sexes and all ages were affected, though signs were not
noted in sheep younger than five months. The sheep had been grazing
for several months on kikuyu pastures in the mornings and paddocks
of Phalaris grass and another unspecified grass type in the afternoons.
The specific Phalaris cultivar was not identified, as the owner
did not wish to incur further expenses. The sheep were regularly
drenched with anthelmintics and had been inoculated with a multivalent
Clostridium/Pasteurella vaccine. A live, five-month-old ram exhibiting
a stiff gait and periodic convulsions was presented for necropsy.
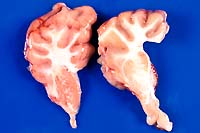
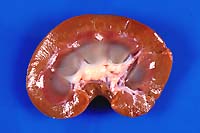
- Case 21-2. Gross Images (The lesions described below
are very subtle in these photos.)
Gross Pathology: The most striking lesions were diffuse
brown discoloration of the cerebral and cerebellar gray matter,
thalamus, brain stem and medulla; dark brown renal cortices;
and diffuse olive green to bluish discoloration of the renal
medullae. Incidental lesions included mild hydrothorax and hydropericardium,
and low numbers of wire-, nodular-, and tapeworms were present.
After formalin fixation, scattered multifocal dark brown to black
specks, probably representing pigmented nuclei, were evident
in the brain stem and medulla.
Laboratory Results: Liver and kidney copper values were
within normal limits.
-
- Contributor's Diagnosis and Comments: Brain stem/medulla:
Neuronal cytoplasmic lipofuscin pigmentation, multifocal, moderate.
-
- A section of medulla or brain stem is submitted for examination.
Scattered pigmented neurons, usually involving specific nuclei,
are evident. Affected neurons are either mildly swollen or slightly
shrunken and basophilic. The pigment is present loosely dispersed
perinuclearly as golden or dark brown pigment granules, or more
commonly in a distinct clump to the one side of the nucleus.
The granules were strongly positive for lipofuscin with the Schmorls'
stain, but negative for hemosiderin (Prussian blue stain). Mild
widening of the perineuronal and perivascular spaces, probably
attributable to edema, is evident. Isolated glial cells in the
neuropil between affected neurons reveal nuclear pyknosis. Other
lesions (tissues not submitted) included moderate pigmentation
of the ventral spinal motor neurons, mild status spongiosis of
the spinal cord white matter, mild multifocal pigmentation of
renal cortical and medullary epithelial cells, and scattered
renal medullary lipofuscin casts.
-
- Neuronal lipofuscinosis has been described in sheep in South
Africa in outbreaks of Phalaris staggers and Trachyandra divericata
poisoning, and in individual aged sheep.1,5 The case presented
is typical for the chronic form of Phalaris poisoning referred
to as Phalaris staggers. Phalaris poisoning in sheep is reported
to occur in three forms: a "sudden death" syndrome;
acute Phalaris poisoning associated with transient nervous signs;
and a chronic form referred to as Phalaris staggers.1 Bourke
et al. proposed that Phalaris poisoning presents as two syndromes:
an acute syndrome (the so-called "sudden death" syndrome)
characterized by sudden development of neurological signs in
the absence of microscopic changes in the central nervous system
(CNS); and a chronic syndrome (i.e. Phalaris staggers) characterized
by gradual development of neurological signs and the presence
of characteristic lesions in the CNS.4 The latter form is a slowly
progressive, irreversible, neurological disorder which occurs
in sheep several weeks to months after grazing on Phalaris pastures.1,4
The nervous signs are thought to be due to 3-tryptamine alkaloids
which are structurally similar to serotonin.1 No reference could
be found as to the relationship between these alkaloids and the
development and accumulation of the lipofuscin-type pigment.
-
- The "sudden death syndrome" was previously believed
to be due to sudden cardiac arrest, but recent evidence suggests
that it can occur as one of two presentations: a cardiac presentation
or polioencephalomalacic (PEM) presentation. At least four different
underlying mechanisms are thought to be responsible for this
syndrome: cardio-respiratory toxins (possibly phenylethylamines
and some related chemical structures); a thiaminase; cyanogenic
compounds; and nitrate compounds. The cardiac presentation occurs
uncommonly and is characterized by cardiac arrhythmias and tachycardia
followed by ventricular fibrillation. The PEM form is characterized
histologically by typical lesions of thiaminase-associated PEM.
-
- Phalaris poisoning is a well-known condition in Australia
and New Zealand where it has been attributed mainly to the perennial
grass Phalaris aquatica and, to a lesser extent, the annual grass
P. minor. In South Africa, only sporadic outbreaks have been
reported in the Western Cape attributable to P. minor.1,2 The
present outbreak is the only one that has been reported outside
the Western Cape.
-
- The principal differential diagnosis for neuronal pigmentation
of sheep in southern Africa includes old age pigmentation in
individual aged sheep (reportedly not evident in sheep <3
years)5 and poisoning due to the ingestion of tumble-weed (Trachyandra
laxa and T. divaricata). In the latter condition, pigmentation
is only rarely evident grossly as occasional khaki-brown flecks
in scattered nuclei of the brain stem and/or in the spinal cord
gray matter. Definitive differentiation between Phalaris staggers
and Trachyandra poisoning rests on pasture/veld investigation
for the presence of Phalaris or Trachyandra plants.
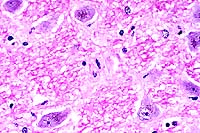 40x
obj
40x
obj
- Case 21-2. Brainstem. Neurons contain granular brown
perinuclear pigment.
-
- AFIP Diagnosis: Brain stem, neurons: Brown granular
pigmentation, perinuclear, multifocal, moderate, Ile de France,
ovine.
-
- Conference Note: Phalaris and Trachyandra sp. plants
are known to induce intense lipofuscin storage in neurons of
the thalamus, spinal cord, and peripheral ganglia, although the
toxic principle and pathogenesis remain to be identified. While
the observation of the pigment in neurons is diagnostically useful
in suspected cases of toxicity, especially in young animals,
the accumulation of lipofuscin does not seem sufficient to explain
the clinical signs and mortality. As noted by the contributor,
some authors have proposed that central nervous system signs
result from serotonergic effects of toxins on upper motor neurons.
The presence of lipofuscin may represent accumulation of indolic
metabolites in neuronal lysosomes.
-
- Other conditions causing excessive accumulation of lipopigments
within neurons discussed by conference participants included
Gomen disease of horses and inherited ceroid-lipofuscinosis.
Gomen disease occurs in horses in New Caledonia, and a toxin
is suspected as the underlying etiology. Gomen disease is characterized
by cerebellar neuronal degeneration, loss of Purkinje and granule
cells, thinning of the molecular layer, and accumulation of lipofuscin
in surviving Purkinje cells and neurons of the brain and spinal
cord. Inherited ceroid-lipofuscinosis is due to an autosomal
recessive trait reported in sheep, cattle, dogs, and cats. Neurons
at all levels of the brain and spinal cord contain lipopigments,
and many other cell types are frequently affected as well.
-
- Contributor: Onderstepoort Veterinary Institute, Pathology
Section, PO Box 12502, Onderstepoort 0110, South Africa.
-
- References:
- 1. Kellerman TS, Coetzer JAW, Naudé TW: Plant poisonings
and mycotoxicoses of livestock in southern Africa, 2nd ed., pp.
24-28, Oxford University Press, Cape Town, South Africa, (in
print).
- 2. Van Halderen A, Green JR, Schneider DJ: An outbreak of
suspected Phalaris staggers in sheep in the Western Cape Province.
J South African Vet Assoc 61:39-40, 1990.
- 3. Bourke CA, Carrigan MJ: Mechanisms underlying Phalaris
aquatica "sudden death" syndrome in sheep. Australian
Vet J 69:165-167, 1992.
- 4. Bourke CA, Carrigan MJ, Seaman JT, Evers JV: Delayed development
of clinical signs in sheep affected by Phalaris aquatica staggers.
Australian Vet J 69:31-32, 1987.
- 5. Newsholme SJ, Schneider DJ, Reid C: A suspected lipofuscin
storage disease of sheep associated with ingestion of the plant,
Trachyandra divaricata. Onderstepoort J Vet Res 52:87-92, 1985.
- 6. Jubb KVF, Huxtable CR: The nervous system. In: Pathology
of Domestic Animals, Jubb KVF, Kennedy PC, Palmer N, eds., 4th
edition, vol. 1, pp. 285 and 317-321, Academic Press, San Diego,
CA, 1993.
- 7. Summers BA, Cummings JF, de Lahunta A: Degenerative diseases
of the central nervous system. In: Veterinary Neuropathology,
page 263, Mosby Yearbook, St. Louis, MO, 1995.
- 8. Jolly RD, Walkley SU: Lysosomal storage diseases of animals:
An essay in comparative pathology. Vet Pathol 34:527-548, 1997.
-
Case III - 98-1263 (AFIP 2638861)
- Signalment: Sixteen-month-old, female, Dorset sheep.
-
- History: The ewe was noted to be off feed in the morning
and was dead when checked again in the early afternoon of the
same day. No previous signs were noted.
-
- Gross Pathology: The ewe was moderately obese. Petechiae
were seen on the visceral pleura lining the thoracic cavity and
paralleling the coronary vessels. The liver was firm and had
an accentuated lobular pattern.
-
- Laboratory Results: The liver copper level was 333
mg/g (toxic range is greater than 250 mg/g), and the kidney copper
level was 149 mg/g (toxic range is greater than 18 mg/g).
-
- Contributor's Diagnoses and Comments:
- 1. Hepatocellular necrosis, centrilobular, severe, acute,
liver.
2. Periportal fibrosis, biliary hyperplasia, and occasional megalocytosis,
liver.
-
- The liver has severe, nearly massive, centrilobular and midzonal
hepatocellular necrosis together with severe periportal to bridging
fibrosis and biliary hyperplasia. Approximately 10-15% of the
hepatocytes remain. In addition to necrosis, most centrilobular
regions have severe hemorrhage. Some hepatocytes that remain
in periportal zones are large and have big, vesicular nuclei
(karyomegaly). There is fibrosis and biliary hyperplasia that
bridges portal areas. Fibrosis breaches the limiting plates and
extends into hepatic lobules. The periportal zones have mild
to moderate sized accumulations of macrophages admixed with a
few lymphocytes. Many of the macrophages have pale, brown cytoplasmic
pigment.
-
- Intratubular hemoglobin casts were observed in the kidney
in addition to the hepatic changes. The acute hepatocellular
necrosis, taken with the kidney lesions, suggests acute hemolytic
crisis precipitated by copper intoxication. This is supported
by the elevated liver and kidney copper levels. The periportal
fibrosis, biliary hyperplasia, and megalocytosis suggest an underlying
pyrrolizidine alkaloid toxicosis or aflatoxicosis. Pyrrolizidine
alkaloids are the most significant hepatotoxins associated with
chronic copper poisoning, with Heliotropium or Echium sp. being
the most common. Pyrrolizidine alkaloid inhibition of hepatocyte
mitosis, which decreases the ability of the liver to replace
hepatocytes lysed by excessive copper, is one possible mechanism.
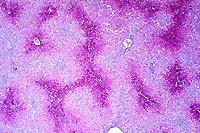 2x
obj.
2x
obj.
- Case 21-3. Liver. There is diffuse centrilobular to
submassive hepatocellular necrosis.
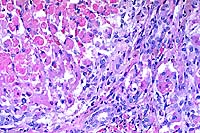 20x
obj.
20x
obj.
- Case 21-3. Liver. There is proliferation of periportal
fibroblasts and bile duct epithelial cells, with adjacent necrotic
hepatocytes.
-
- AFIP Diagnoses:
- 1. Liver: Necrosis, centrilobular to submassive, with hemorrhage
and intrahistiocytic light greenish-brown pigment, Dorset sheep,
ovine.
2. Liver: Biliary hyperplasia and bridging portal fibrosis, diffuse,
mild to moderate.
-
- Comment: Some participants observed intranuclear inclusions
which were considered to be non-viral. Participants identified
biliary hyperplasia and bridging portal fibrosis, but did not
identify hepatocyte megalocytosis. The rhodanine stain performed
at the AFIP revealed moderate amounts of intracytoplasmic copper
within Kupffer cells in portal areas and small amounts within
periportal hepatocytes.
-
- Conference Note: Copper is unique among the heavy
metals for its selective toxic effects on the liver. Of the domestic
animal species, sheep are the most prone to copper poisoning.
The avidity of the liver for copper, coupled with the limited
rate of copper excretion in the bile in sheep, predispose these
animals to chronic copper toxicity. Additionally, chronic copper
poisoning in sheep is related to three environmental factors:
excessive copper intake, such as from contaminated pasture; increased
bioavailability of dietary copper due to low molybdenum levels
resulting in excessive absorption and accumulation (molybdenum
forms insoluble complexes with copper in the gastrointestinal
tract); and the presence of other hepatotoxins, such as pyrrolizidine
alkaloids, predisposing animals to outbreaks.
-
- In the absence of contributory factors, liver copper concentrations
less than 200-300 parts per million (ppm) on a dry matter basis
seem to cause little hepatocellular damage in sheep. Microscopic
changes occur in the liver at levels of 300 ppm or more, observed
initially as single cell hepatocyte apoptosis and neutrophilic
inflammation. The apoptotic rate increases as the level of copper
accumulation increases, and the mitotic rate simultaneously increases
to keep pace with lost hepatocytes. Sheep with liver concentrations
in excess of 1,000 ppm may be clinically and hematologically
normal, if the mitotic rate produces adequate numbers of new
hepatocytes to take up copper released by dying liver cells.
When hepatocellular loss exceeds the rate of replacement, copper
spills into the plasma in high enough concentrations to damage
erythrocytes, causing intravascular hemolysis, which further
accelerates hepatocellular necrosis. Thus, hepatocellular mitotic
activity is critical to the delay of acute hemolytic crises.
-
- Pyrrolizidine alkaloids, found in a variety of toxic plants,
are well-known for their antimitotic effect on hepatocytes. The
interference of hepatocellular replication leads to acute hemolytic
crisis at an earlier stage of copper accumulation in affected
sheep. This mechanism probably explains the marginal elevation
in liver copper levels of the clinically affected sheep submitted
for examination. Less specific stresses, such as brief periods
of starvation, may also precipitate a hemolytic crisis. Histologically,
pyrrolizidine alkaloid hepatotoxicity is characterized by hepatocyte
megalocytosis, with concurrent biliary hyperplasia and portal
fibrosis. The severity of portal fibrosis is species variable,
being minimal to mild in sheep, moderate in horses, and severe
in cattle, in which veno-occlusive disease may be observed.
-
- Conference participants did not initially attribute the microscopic
lesions in the liver of this sheep to chronic copper toxicosis
and pyrrolizidine alkaloid exposure. Megalocytosis is a consistent
microscopic feature in cases of pyrrolizidine alkaloid toxicosis,
and participants did not consider this a prominent finding in
the examined sections. Biliary hyperplasia and portal fibrosis
are relatively nonspecific changes. Chronic hepatic disease of
various causes may secondarily lead to abnormal accumulation
of copper in the liver. Thus, participants found it difficult
to determine the pathogenesis of the liver lesions and accumulation
of copper. After learning of the laboratory and other pathologic
findings, participants agreed with the contributor's assessment
of this case.
-
- Contributor: Department of Veterinary Microbiology
and Pathology, Washington State University, Pullman, WA 99164-7040.
-
- References:
- 1. Howell JH, et al.: Experimental copper and Heliotrope
intoxication in sheep: Morphological changes. J Comp Pathol 105:49-74,
1991.
- 2. Seaman JT: Hepatogenous chronic copper poisoning in sheep
associated with grazing Echium plantagineum. Australian Vet J
62:247-248, 1985.
- 3. Seaman JT: Pyrrolizidine alkaloid poisoning of sheep in
New South Wales. Australian Vet J 64:164-167, 1987.
- 4. Jones TC, Hunt RD, King NW: Diseases due to extraneous
poisons. In: Veterinary Pathology, 6th ed., pp. 708 & 712-718,
Williams and Wilkins, Baltimore, MD, 1997.
- 5. Jones TC, Hunt RD, King NW: The digestive system. In:
Veterinary Pathology, 6th ed., pp. 1098-1100, Williams and Wilkins,
Baltimore, MD, 1997.
- 6. Kelly WR: The liver and biliary system. In: Pathology
of Domestic Animals, Jubb KVF, Kennedy PC, Palmer N, eds., 4th
edition, volume 2, pp. 392-400, Academic Press, San Diego, CA,
1993.
-
Case IV - ND1 (AFIP 2641844)
- Signalment: Three-month-old Hereford heifer.
-
- History: Several calves tore down a fence around a
lagoon overflow pond. A total of three calves were found dead
near the lagoon. A heavy algal growth was noted on the lagoon
surface.
-
- Gross Pathology: Per the referring veterinarian, petechiation
of serosal surfaces of abdominal organs was present. Only the
liver was submitted to the diagnostic laboratory. On cut surface,
coalescing areas of hemorrhage were noted throughout the hepatic
parenchyma.
-
- Laboratory Results: A water sample from the lagoon
submitted to the North Dakota Department of Health identified
pure growth of Microcystis aeruginosa.
Contributor's Diagnosis and Comments: Liver: Centrilobular
necrosis and hemorrhage, severe, acute, coalescing with centrilobular
hepatocellular disassociation.
Etiology: Toxicosis due to Microcystis aeruginosa ("blue-green
algae") and microcystin-LR toxin.
-
- Microcystin-LR, a hepatotoxin produced by the blue-green
algae Microcystis aeruginosa, sporadically causes sudden death
in livestock. Typical case histories indicate lagoons or ponds
with a heavy algal bloom that is ingested by drinking livestock.
The ultrastructural lesion, occurring as early as ten minutes
after ingestion, is a rearrangement of the actin cytoskeleton
leading to cell rounding, disassociation, and degeneration. Necrosis
of hepatocytes is observed at 60 minutes. Light microscopic changes
include centrilobular necrosis and hemorrhage, as seen in this
case. Differential diagnosis included bovine pestivirus (bovine
viral diarrhea), Clostridium chauvoei, salmonellosis, lead toxicosis,
and lightning strike.
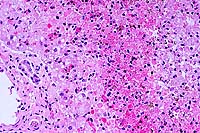 20x
obj.
20x
obj.
- Case 21-4. Liver. There is degeneration, individualization,
and necrosis of hepatocytes throughout the lobule with centrilobular
hemorrhage and hemosiderin deposition.
-
- AFIP Diagnosis: Liver: Necrosis, massive, with hepatocellular
disassociation and hemorrhage, Hereford, bovine.
-
- Conference Note: Several species of blue-green are
known to cause poisoning in domestic and wild animals or birds
in various parts of the world. Most documented cases involved
Microcystis aeruginosa, though other species known to cause toxicity
include members of the genera Anabaena, Oscillatoria, and Nostoc.
While outbreaks of blue-green algae poisoning are uncommon, they
can be responsible for high mortality. The algal blooms have
been associated with water runoff from fertilized soil, and the
algae may then become concentrated on the shoreline of ponds
due to the effects of wind. Poisoning occurs most commonly in
ruminants, although cases have also been reported in horses,
dogs, sheep, swine, and domestic poultry.
-
- In most poisoned animals, death occurs within hours of ingestion
of contaminated water, and few animals recover. A few cases may
have a subacute or chronic clinical course. When observed, clinical
signs in acute cases include prostration followed by convulsions,
or generalized paralysis. Generalized petechiation and cavitary
effusions are observed at necropsy in acute cases. In subacute
and chronic cases, clinical signs reflect hepatic insult and
include ataxia, depression, anorexia, hemorrhagic diarrhea, and
icterus. Necropsy findings in subacute cases include icterus
and a fatty, yellow, friable liver. In chronic cases, the liver
may be hard and cirrhotic; severe cutaneous lesions caused by
photosensitization may be observed in recovered animals.
-
- Freshwater blue-green algae produce a variety of hepatotoxins
known as microcystins that are inhibitors of protein phosphatases.
The toxic principle, microcystin-LR, is released upon disintegration
of algae either in the water, or within the rumen or stomach
after ingestion. Microcystin-LR is one of the most potent hepatotoxins
and produces coagulative necrosis and hemorrhage in the liver.
Covalent binding of protein phosphatases by microcystin-LR inhibits
the activity of the enzymes and leads to hyperphosphorylation
of cytoskeletal proteins, rearrangement of intermediate filaments
and microtubules, and disorganization of the cytoskeleton, resulting
in disassociation and necrosis of hepatocytes. Microcystin-LR
also causes necrosis of endothelial cells in the liver and may
result in embolization of hepatocytes to the lung. Recently,
some investigators have attributed the extensive coagulative
necrosis in the liver to ischemia based upon the distribution
of the microscopic lesions in the early stages of experimentally
induced toxicity in rodents. Both mechanisms probably contribute
to the severity of the hepatic lesion. Microcystins also have
tumor promoting activity in animals.
-
- Contributor: North Dakota State University, Veterinary
Diagnostic Laboratory, Fargo, ND 58105.
-
- References:
- 1. Hooser SB, et al.: Microcystis-LR-induced ultrastructural
changes in rats. Vet Pathol 27:9-15, 1990.
- 2. Hooser SB, et al.: Actin filament alternatives in rat
hepatocytes induced in vivo and in vitro by microcystin-LR, a
hepatotoxin from the blue-green alga, Microcystis aeruginosa.
Vet Pathol 28:259-266, 1991.
- 3. George LW: Diseases of the nervous system. In: Large Animal
Internal Medicine: Diseases of Horses, Cattle, Sheep, and Goats,
Smith BP, ed., 2nd edition, pp. 1078-1080, Mosby-Year Book, St.
Louis, MO, 1996.
- 4. Kelly WR: The liver and biliary system. In: Pathology
of Domestic Animals, Jubb KVF, Kennedy PC, Palmer N, eds., 4th
edition, volume 2, pp. 385-386, Academic Press, San Diego, CA,
1993.
- 5. Jones TC, Hunt RD, King NW: Diseases due to extraneous
poisons. In: Veterinary Pathology, 6th ed., pp. 723-724, Williams
and Wilkins, Baltimore, MD, 1997.
- 6. Yoshida T, et al.: Immunohistochemical localization of
microcystin-LR in the liver of mice: A study on the pathogenesis
of microcystin-LR-induced hepatotoxicity. Toxicol Pathol 26:411-418,
1998.
-
-
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
- Return to WSC Case Menu
 20x
obj.
20x
obj.
 40x
obj.
40x
obj.


 40x
obj
40x
obj
 2x
obj.
2x
obj.
 20x
obj.
20x
obj.
 20x
obj.
20x
obj.