Results
AFIP Wednesday Slide Conference - No. 14
December 14, 1998
- Conference Moderator:
LTC Michael J. Topper
Walter Reed Army Institute of Research
Division of Pathology
Washington, D.C. 20307
NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
- Case I - 98-4779 (AFIP 2638822)
-
- Signalment: Tissue from a two-day-old, male, quarter
horse.
-
- History: This horse presented to the veterinary teaching
hospital with contracted flexor tendons in all limbs and prognathism.
Radiographic examination of the metacarpal bones showed incomplete
ossification. The foal was euthanized and necropsied.
-
- Gross Pathology: The carpal joints had an angle of
140° at full extension. The thyroid gland was grossly normal.
-
- Laboratory Results: No antemortem blood tests were
performed. Liver selenium levels were deficient (0.11 mg/g wet
weight). Adequate liver selenium range is between 0.200 - 0.600
mg/g wet weight.
-
- Contributor's Diagnosis and Comments: Congenital thyroid
hyperplasia with secondary flexural limb deformities and prognathism.
-
- In sections of both lobes of the thyroid gland, follicles
are small and lack colloid. Follicular epithelial cells are columnar
to polygonal and occasionally bilayered. The cytoplasm is eosinophilic,
abundant, and contains large, clear, poorly defined vacuoles.
Basal to central nuclei are large (up to 15 mm in diameter),
round and have coarsely stippled chromatin.
-
- The histologic changes in the thyroid gland, including lack
of colloid in follicles in conjunction with hypertrophy and hyperplasia
of the follicular epithelial cells, and the clinically noted
delayed ossification and contraction of the limbs are consistent
with a syndrome described in newborn foals called thyroid hyperplasia
with concurrent musculoskeletal deformities (TH-MSD).1,2 Similar
lesions have also been described in aborted equine fetuses.3
The cause of TH-MSD is unknown. Males and females are affected
equally, and the syndrome has been documented in eight different
breeds making a genetic predisposition unlikely. Many cases originate
from farms where the syndrome has been present in other foals
during the same or other foaling seasons, suggesting that a dietary
deficiency, toxic substance or infectious agent is responsible.
Similar lesions have been reported in foals from mares consuming
feeds contaminated with fungi (Acremonium coenophialum and Claviceps
purpurea) or locoweed (Astragalus mollisimus).
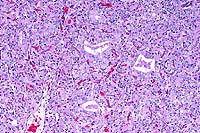 10x
obj
10x
obj
- Case14-1. Thyroid. There is diffuse follicular epithelial
hypertrophy and hyperplasia. Relatively few follicles have discernable
lumens or colloid production.
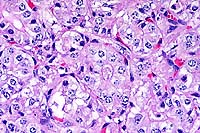 40x
obj
40x
obj
- Case14-1. Thyroid. Follicular epithelium is hypertrophic
and vacuolated. Follicle colloid is globular and sparce.
-
- AFIP Diagnosis: Thyroid gland: Hyperplasia, follicular,
diffuse, severe, quarter horse, equine.
-
- Conference Note: Goiter, defined as non-neoplastic
and noninfectious enlargement of the thyroid gland, is a common
presentation of thyroid disease. Goiter may be diffuse or multinodular,
and hyperplastic or colloidal. Development of goiter has been
associated with iodine deficient diets, ingestion of goitrogens
that interfere with thyroid hormone synthesis, excess dietary
iodide, and genetic enzyme defects involved in thyroid hormone
biosynthesis.
-
- Impairment of thyroid hormone production leads to hypothyroidism
and compensatory secretion of thyroid stimulating hormone (TSH)
from the pituitary gland, increased serum TSH levels, and hypertrophy
and hyperplasia of thyroid follicular cells with the resultant
gross enlargement of the gland. The severity of glandular enlargement
is related to the level and duration of deficiency of thyroid
hormone.
-
- In animals, congenital hypothyroidism is almost always associated
with hyperplastic goiter and results when inadequate maternal
thyroid hormone crosses the placental barrier during development
in utero. The fetal pituitary gland responds by secretion of
TSH, resulting in fetal hyperplastic goiter. The dam may not
have signs of thyroid gland dysfunction. Dystocia, retained placenta,
and prolonged gestation have also been associated with congenital
hypothyroidism in newborn animals. In horses, affected foals
are born extremely weak and die within a few days of birth. The
thyroid gland may be only slightly enlarged in these cases. Calves,
piglets, lambs, and kids are also susceptible to congenital hypothyroidism
to varying degrees. Myxedema and a visibly enlarged thyroid gland
are more common in these species compared to the horse. In carnivores,
congenital hyperplastic goiter is not a common feature of hypothyroidism,
though congenital thyroid enlargement in puppies has been reported
and may result in fetal asphyxiation and dystocia.
-
- In humans, hypothyroidism occurring during infancy or early
childhood is termed cretinism. Cretinism results from the severe
neurological deficits and central nervous system malformations
that occur if the fetus is deprived of maternally derived thyroid
hormones during critical periods of in utero brain development.
Other clinical features of cretinism include impaired development
of the skeletal system, protrusion of the tongue, and umbilical
hernia.
-
- Skeletal deformities in humans include severe dwarfism, delayed
appearance of deciduous teeth, lack of closure of fontanels of
the skull, and delayed closure of the epiphyses. These skeletal
deformities are due to defects in cartilage maturation. The maturation
of the zone of cartilage hypertrophy is delayed, and the zone
of cartilage proliferation is narrowed, resulting in disorderly
progression and failure of proper endochondral ossification.
Disturbances and delays in endochondral ossification in the horse
most often affect the carpal bones, and failure of proper ossification
of the carpal and tarsal cuboidal bones may lead to angular limb
deformities in foals. The angular limb deformities observed in
this foal are the result of defects in endochondral ossification
secondary to congenital hypothyroidism.
-
- Contributor: Department of Veterinary Microbiology
and Pathology, Washington State University, Pullman, WA 99164-7040.
- References:
- 1. Doige CE, McLaughlin BG: Hyperplastic goitre in newborn
foals in western Canada. Can Vet J 22:42-45, 1981.
- 2. Allen AL, Doige CE, Fretz PB, Townsend HGG: Hyperplasia
of the thyroid gland and concurrent musculoskeletal deformities
in western Canadian foals: Reexamination of a previously described
syndrome. Can Vet J 35:31-38, 1994.
- 3. Allen AL: Hyperplasia of the thyroid gland and musculoskeletal
deformities in two equine abortuses. Can Vet J 36:234-236, 1995.
- 4. Capen CC: The endocrine glands. In: Pathology of Domestic
Animals, Jubb KVF, Kennedy PC, Palmer N, eds., 4th ed., vol.
3, pp. 315-321, Academic Press, 1993.
- 5. Palmer N: Bones and joints. In: Pathology of Domestic
Animals, Jubb KVF, Kennedy PC, Palmer N, eds., 4th ed., vol.
1, pp. 22-24, Academic Press, 1993.
- 6. Cotran RS, Kumar V, Collins T: The endocrine system. In:
Robbins Pathologic Basis of Disease, 6th ed., pp. 1132-1140,
WB Saunders, Philadelphia, PA, 1999.
- 7. Schiller AL: Bones and joints. In: Pathology, Rubin R,
Farber JL, eds., page 1315, JB Lippincott Co., Philadelphia,
PA, 1988.
-
-
- Case II - 4435-98 (AFIP 2643729)
-
- Signalment: Seven-month-old, female, Yorkshire Terrier.
-
- History: The dog was slobbering and bewildered on
presentation to the veterinarian.
-
- Gross Pathology: A prominent vascular shunt was identified
in the liver by the veterinarian at surgery.
- Laboratory Results: Alkaline phosphate 184; SGPT 100; SGOT
108; Total Bilirubin 0.2; Pre-prandial bile acids: 3.9 mmol/L
(normal is less than 5); Post-prandial bile acids: 329 (normal
is less than 15).
-
- Contributor's Diagnosis and Comments: Portosystemic
vascular shunt, congenital.
-
- The surgical biopsy of the liver shows essentially no portal
vein branches, but there is mild reduplication of the arterioles
in the portal areas. The lobules seem small as evidenced by a
subjective decrease in distance between portal triads.
-
- A prominent shunt was identified by the surgeon at the time
of biopsy. Corrective surgery was performed several weeks later.
A ring was installed that closed the shunt over a three week
period, and the dog recovered completely and was normal one month
post-surgery. In cats and small breed dogs, the shunts are usually
extra-hepatic and occur between the portal vein and caudal vena
cava or azygous vein. In large breed dogs, intra-hepatic shunts
are more common and occur as a result of a patent ductus venosus.
Congenital aplasia/hypoplasia of the portal vein with secondary
collateral circulation development and subsequent portosystemic
shunting has also been described in dogs.
- Shunts cause the portal circulation to bypass the liver and
enter the systemic circulation. The lack of portal circulation
leads to a small liver with small hepatocytes because the stimulating
factors such as insulin, glucagon, and amino acids are decreased.
The portal vein branches in the liver are very small or absent.
-
- The decreased functional liver mass is reflected in the increase
in the post-prandial blood bile acids level. Bile acids are produced
in the liver; the organ has a tremendous reserve capacity for
bile acid production, but little reserve capacity for its conjugation
and excretion. Pre-prandial bile acids are usually also increased
with hepatic insufficiency, but can be normal after a prolonged
fast. The hepatic lobules from the liver of this Yorkshire terrier
were 40-50% smaller (decreased distance between portal triads)
when compared to a liver from a normal three-month-old Yorkshire
terrier. Acquired shunts may result from chronic hypertension
and may be accompanied by ascites.
-
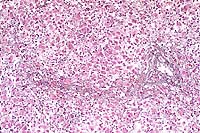
 20x
obj
20x
obj
- Case14-2. Liver. Somewhat serpentine arrangements
of arteriolar smooth muscle cells represent portal arteriolar
hyperplasia. Portal veins are not discernable. Scattered hepatocytes
contain brown pigment.
-
- AFIP Diagnosis: Liver: Arteriolar hyperplasia, portal,
diffuse, moderate, with portal vein hypoplasia and lobular atrophy,
Yorkshire terrier, canine.
-
- Conference Note: Portosystemic shunts are communications
between the portal and systemic vasculature that allow passage
of portal blood to the systemic circulation without first passing
through the liver; shunts may be acquired or congenital. Congenital
vascular shunts occur more often in dogs, less frequently in
cats, and sporadically in other domestic animals including calves,
foals, and pigs. Acquired shunts most often form as a result
of compensatory development of collateral vessels in response
to sustained portal hypertension caused by severe diffuse hepatic
disease, such as chronic hepatitis and cirrhosis. Acquired shunts
are usually multiple and occur as a tortuous plexus of vessels
that communicate with the perirenal caudal vena cava. Rarely,
young dogs may have arteriovenous (arterioportal) fistulae and
develop portal hypertension, ascites, and acquired shunts.
-
- Animals with portosystemic shunts are often small for their
age and breed, and present in marginal or poor nutritional condition.
The animal is frequently presented to the veterinarian for signs
of hepatoencephalopathy (HE), including disorientation, hypersalivation,
aggression, ataxia, blindness, and seizures. Central nervous
system (CNS) signs typically wax and wane and are exacerbated
by meals or high protein diets.
-
- The pathogenesis of HE is probably multifactorial, and the
condition results from inadequate clearance of enterically derived
toxins in portal blood, including ammonia, mercaptans, short-chain
fatty acids, and gamma aminobutyric acid. During periods of hyperammonemia,
ammonia crosses the blood-brain barrier and is directly toxic
to astrocytes. Furthermore, astrocytes metabolize ammonia to
glutamine, which is also thought to be neurotoxic. Increased
blood levels of amino acids, including tryptophan, phenylalanine,
and tyrosine, readily reach the CNS due to changes in the blood-brain
barrier in HE. Tryptophan in particular is toxic to the CNS,
while tyrosine can give rise to octopamine which can act as a
pseudotransmitter. Increased synthesis and absorption of gamma-aminobutyric
acid (GABA) by bacteria occurs in the gut in animals with portosystemic
shunts; this powerful inhibitory neurotransmitter may disrupt
the balance of neuronal excitation and inhibition.
- Gross neuropathological changes are not present in animals
with HE. While not specific for HE, microscopic changes in the
brain include spongiform change or polymicrocavitation of the
white matter and the presence of Alzheimer type II cells. Alzheimer
type II cells occur as small clusters of astrocytes with swollen,
clear nuclei. In HE, spongiform changes occur diffusely in the
white matter and are bilateral and symmetrical in distribution.
- In addition to neurological signs, animals with shunts may
suffer from renal, cystic, or urethral calculi due to increased
urinary excretion of ammonia and uric acid with the resultant
formation of ammonium biurate crystals, especially in alkaline
urine. The urate calculi are usually green. In addition to the
derangements in bile acids noted by the contributor, animals
with portosystemic shunts often have hypoalbuminemia in the absence
of proteinuria, low blood urea nitrogen, hypoglycemia, and hypocholesterolemia;
the decrease in these serum chemistry values reflects reduced
hepatic synthesis and metabolism of these compounds due to decreased
functional hepatic mass. Erythrocytic microcytosis is a common
finding in animals with portosystemic shunts; the cause is unknown,
but it is not related to iron deficiency.
-
- Contributor: Arkansas Livestock and Poultry Commission,
#1 Natural Resources Drive, Little Rock, AR 72205.
-
- References:
- 1. Center SA: Biochemical evaluation of hepatic function
in the dog and cat. In: Current Veterinary Therapy IX, Kirk RW,
ed., pp. 924-936, WB Saunders Co., Philadelphia, PA, 1986.
- 2. Center SA, Hornbuckle WE: Congenital portosystemic shunts
in cats. In: Current Veterinary Therapy IX, Kirk RW, ed., pp.
825-836, WB Saunders Co., Philadelphia, PA, 1986.
- 3. Kelly, WR: The liver and biliary system. In: Pathology
of Domestic Animals, Jubb KVF, Kennedy PC, Palmer N, eds., vol.
2, pp. 323-324, Academic Press, San Diego, CA, 1993.
- 4. Summers BA, Cummings JF, de Lahunta A: Degenerative diseases
of the central nervous system: Metabolic and circulatory disorders.
In: Veterinary Neuropathology, pp. 208-211, Mosby Yearbook, St.
Louis, MO, 1995.
5. Johnson SE, Sherding RG: Diseases of the liver and biliary
tract. In: Manual of Small Animal Practice, Birchard SJ, Sherding
RG, eds., pp. 751-753, WB Saunders, Philadelphia, PA, 1994.
-
-
- Case III - 97-1455 (AFIP 2643519)
-
- Signalment: Four-year-old, castrated male, Cocker
Spaniel.
-
- History: The dog presented to the Veterinary Teaching
Hospital at North Carolina State University with a two week history
of diarrhea, melena and ascites. Clinical blood count (CBC) and
chemistry data revealed a macrocytic anemia, thrombocytopenia,
leukocytosis, neutrophilia with regenerative left shift, monocytosis,
eosinopenia, hypoproteinemia, hypoalbuminemia, azotemia, hyperphosphatemia,
hypernatremia, hyperkalemia and hypochloremia. Urinalysis was
normal, and the specific gravity was 1.018. Ultrasonography of
the abdomen revealed increased hepatic arterial blood flow and
decreased portal blood flow of 0.06 m/s (normal 0.15-0.20) into
the liver. The dog failed to respond to supportive therapy over
the following two weeks and was euthanized.
Gross Pathology: The abdomen contained three liters of
light yellow, clear, watery fluid. The liver was small, tan,
firm, had a coarse, granular surface, and comprised 1.5% of the
body weight. There were 5-10 white, firm nodules, ranging from
3-7 millimeters in diameter, on the liver surface. Engorged mesenteric
veins drained from the duodenum into the caudal vena cava.
-
- Laboratory Results:
-
- Clinical Blood Count:
|
Test |
Results (x1000/ml) |
Normal Range |
|
PCV |
20% |
(33-58) |
|
MCV |
72.9 fl |
(63.6-70.1) |
|
MCHC |
37.6 g/dl |
(33.9-36.7) |
|
Platelet count |
148 |
(181-350) |
|
WBC |
75.4 |
(6.4-15.8) |
|
Mature neutrophils |
67.9 |
(3.0-11.5) |
|
Band neutrophils |
2.3 |
(0.0-0.3) |
|
Monocytes |
3.8 |
(0.15-1.35) |
|
Eosinophils |
0 |
(0.1-0.75) |
|
Aggregate reticulocytes |
6.0% |
(0.0-1.5) |
- Clinical Chemistry:
|
Test |
Result |
Normal Range |
|
Albumin |
1.4 g/dl |
(2.8-3.8) |
|
Alk. phos |
50 IU/L |
(16-71) |
|
ALT |
18 IU/L |
(5-35) |
|
Bilirubin |
0.3 mg/dl |
(0.0-0.5) |
|
BUN |
44 mg/dl |
(6-23) |
|
Ca++ |
7.7 mg/dl |
(8.8-10.7) |
|
Creatinine |
1.3 mg/dl |
(0.9-1.5) |
|
Glucose |
111mg/dl |
(83-122) |
|
Phosphorus |
5.9 mg/dl |
(2.3-5.1) |
|
Total protein |
3.6 g/dl |
(5.5-6.8) |
|
Na+ |
138 mmol/l |
(144-150) |
|
K+ |
5.1 mmol/l |
(3.5-4.7) |
|
Chloride |
108 mmol/l |
(109-118) |
|
Bile acids preprandial |
105.0 umol/l |
(1.0-12.7) |
|
Bile acids postprandial |
321.4 umol/l |
(0.0-15.1) |
Abdominocentesis Results:
The abdominal fluid had a specific gravity of 1.006, protein
0.3 g/dl, and 700 nucleated cells/ml consisting of 84% mature
nondegenerate neutrophils, 15% large mononuclear cells and 2%
lymphocytes. It was characterized as a transudate.
-
- Contributor's Diagnosis and Comments: Lobular dissecting
hepatitis, chronic, diffuse, severe, with minimal nodular regeneration.
-
- The liver is characterized by diffuse dissociation of hepatocytes
including disruption of the limiting plate, thin bands of reticular
and fibrous connective tissue dissecting through sinusoids, a
marked absence of clearly discernible central veins, the presence
of mildly distended portal lymphatics, a mixed inflammatory cell
infiltrate, pigment-laden Kupffer cells, hepatocyte pseudorosettes
and binucleate hepatocytes. The abnormal hepatic lobule morphology
resulted in sinusoidal hypertension and ascites as well as functional
liver deficits, as indicated by elevated serum bile acids. Hepatocyte
injury, however, may not have been a significant component of
this disease, as evidenced by normal serum ALT levels. Other
indications of sinusoidal hypertension were the decreased portal
blood flow and compensatory hepatic arterial blood flow identified
at ultrasound, and engorged mesenteric shunt vessels noted at
necropsy. No prehepatic portal vein nor post hepatic vein thrombi
were found. Although mild endocardiosis was present on the mitral
and tricuspid heart valves, cardiac insufficiency leading to
passive congestion is not thought to play a significant role
in the hepatic changes nor development of ascites in this animal
due to the absence of other signs of right sided heart failure,
such as hepatomegaly, centrilobular congestion, sinusoidal dilation
or atrophy, and hepatocyte vacuolar degeneration or necrosis.
-
- Lobular dissecting hepatitis is a distinct form of hepatitis
seen only in the dog1. It differs in morphology from the syndromes
of chronic active hepatitis, chronic persistent hepatitis, and
chronic lobular hepatitis of man, as well as micro and macronodular
cirrhosis of dogs. It is most similar to a form of human neonatal
hepatitis complex, which is thought to arise from various etiologies
including viruses, toxoplasmosis, Treponema pallidum, metabolic
disturbances, toxins, and idiopathic causes. A specific etiology
has not been identified in prior reports of the disease in dogs,
nor was one found in this case. Lobular dissecting hepatitis
affects young dogs from three months to five years of age, and
has been reported in several breeds including the Rottweiler,
Golden Retriever, Cocker Spaniel and mongrels. Reports of similarly
affected dogs from the same litter and household suggest both
genetic and/or common etiologic sources. The lobular dissecting
reaction pattern appears restricted to the juvenile period, and
it has been suggested that lobular dissecting hepatitis be regarded
as a pattern unique to this age2.
-
- Ancillary tests included rhodanine stain for copper or copper-associated
protein, which was negative in the hepatocytes, trichrome and
reticulin stains. The reticulin stain revealed an increase in
sinusoidal reticulin fibers dissecting between hepatocytes. In
liver cirrhosis, Ito cells have been shown to play an important
role in the deposition of excess collagen and the progression
of fibrosis. It is unknown whether a similar pathogenesis occurs
in lobular dissecting hepatitis.
-
- Interestingly, the cytoplasm of many hepatocytes stained
positively when an immunohistochemical stain against alpha-1-antitrypsin
was applied. Alpha-1-antitrypsin is a protease inhibitor synthesized
by hepatocytes. In man, serum deficiency of this enzyme has been
shown to result from a mutation in the gene encoding alpha-1-antitrypsin.
The mutation causes misfolding of the protein product and its
defective translocation from the endoplasmic reticulum. The mutated
protein accumulates within hepatocytes and can result in pulmonary
and hepatic damage from unregulated tissue proteases. Work by
Sevelius et al. identified a cohort of dogs affected by several
different types of hepatopathies with intrahepatocellular accumulations
of alpha-1-antitrypsin3. Descriptions of liver histopathology
in the affected dogs included several that resembled lobular
dissecting hepatitis. The pathogenesis of alpha-1-antitrypsin
accumulation in canine hepatocytes remains to be elucidated,
but may be linked to unregulated tissue proteases causing hepatic
parenchymal damage.
-
 10x
obj
10x
obj
- Case14-3. Liver. There is diffuse dissociation of
hepatocytes, increased numbers of cells in the sinusioids, and
pale eosinophilic material (collagen) in centrilobular locations.
Central veins are inconspicuous. Kupffer cells contain abundant
pigment.
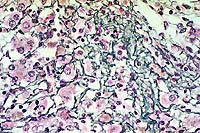
 10x
obj, Reticulin stain
10x
obj, Reticulin stain
- Case14-3. Liver. Reticulin stain demonstrates increased
sinusoidal reticulin extending from portal to centrilobular areas
of the liver.
 40x
obj, Reticulin stain
40x
obj, Reticulin stain
- Case14-3. Liver. This magnification shows abundant
reticulin and individualization of hepatocytes.
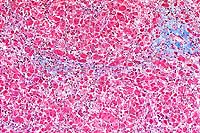 10x
obj, Trichrome stain
10x
obj, Trichrome stain
- Case14-3. Liver. Blue staining areas localize collagen.
-
- AFIP Diagnosis: Liver: Fibrosis, dissecting, diffuse,
moderate, with hepatocellular degeneration and loss, lymphoplasmacytic,
histiocytic, and neutrophilic hepatitis, canulicular cholestasis,
and biliary hyperplasia, Cocker Spaniel, canine.
-
- Conference Note: This case was reviewed by the Department
of Hepatic Pathology. They identified features of subacute necro-inflammatory
injury, delicate intra-acinar centrilobular fibrous strands,
and focal nodular regeneration. They did not identify histologic
features resembling human neonatal hepatitis, such as lobular
disarray with focal hepatocyte necrosis, panlobular or portal
giant cell transformation of hepatocytes, mild mononuclear cell
infiltration of portal areas, and extramedullary hematopoiesis.
As noted by the contributor, lobular dissecting hepatitis is
not a recognized condition in humans. In humans, extreme subacute
hepatic injury may be related to drug-induced hepatotoxicity,
autoimmune hepatitis, and hepatitis of viral etiology. The Department
of Hepatic Pathology found the lesions in this case suggestive
of a toxic injury.
-
- As in humans, most cases of hepatitis in animals are of infectious
or toxic etiology. The specific inciting cause often remains
undetermined, especially in the dog in which chronic progressive
liver disease occurs with some frequency. Chronic hepatitis in
dogs is not a single disease, and various histopathological classifications
taken from human pathology have been applied, including chronic
progressive hepatitis, chronic lobular hepatitis, and chronic
active hepatitis. The etiologies, pathogenesis, and predisposing
genetic factors in chronic canine hepatic disease are not well
understood. Lobular dissecting hepatitis appears to be a distinctive
histopathologic presentation of severe hepatic injury in some
young dogs.
-
- Contributor: North Carolina State University, College
of Veterinary Medicine, 4700 Hillsborough Street, Raleigh, NC
27606.
-
- References:
- 1. Van den Ingh TSGAM, Rothuizen J: Lobular dissecting hepatitis
in juvenile and young adult dogs. J Vet Intern Med 8:217-220,
1994.
- 2. Bennett AM, Davies JD, Gaskell CJ, Lucke VM: Lobular dissecting
hepatitis in the dog. Vet Pathol 20:179-188, 1983.
- 3. Sevelius E, Andersson M, Jönsson L: Hepatic accumulation
of alpha-1-antitrypsin in chronic liver disease in the dog. J
Comp Path 111:401-412, 1994.
- 4. Kelly, WR: The liver and biliary system. In: Pathology
of Domestic Animals, Jubb K, Kennedy P, Palmer N, eds., vol.
2, pp. 361-362, Academic Press, 1993.
- 5. Cotran RS, Kumar V, Collins T: The liver and biliary tract.
In: Robbins Pathologic Basis of Disease, 6th ed., pp. 866-877,
WB Saunders, Philadelphia, PA, 1999.
- 6. Dill-Macky E: Chronic hepatitis in dogs. Vet Clin North
Amer Small Anim Pract 25:387-397, 1995.
-
-
- Case IV - 98 ND2 (AFIP 2642107)
-
- Signalment: Thirteen-month-old, male, llama (Llama
glama).
-
- History: The animal was recently purchased and had
initially done well. The animal then suffered a 2-3 week duration
of respiratory disease that initially responded to antibiotic
therapy. The animal subsequently became anorectic and continued
to lose weight, however. Anthelminthic treatment resulted in
no clinical improvement. Fecal flotation, bacterial culture for
salmonellosis, and Johne's disease agar gel immunodiffusion tests
were performed, but the animal died prior to additional diagnostic
procedures.
-
- Gross Pathology: On necropsy there was marked pulmonary
edema, increased pericardial fluid, and a diffusely pale, mottled
heart. There were multiple coalescing foci of ulceration in the
third compartment (C-3) of the stomach. Scattered, small, 0.2-0.3
cm foci of pallor were present on both the capsular and cut surfaces
of the liver.
-
- Laboratory Results:
-
- Feces:
- Fecal flotation: No parasite eggs identified.
Salmonella sp. bacterial culture: No growth.
Mycobacterium paratuberculosis agar gel immunodiffusion: Negative.
-
- C-3 mucosal ulcerations:
BHV-1 and BVD fluorescent antibody: Negative.
Clostridium perfringens isolated from lesions.
-
- Additional laboratory findings:
Escherichia coli was isolated from the lungs and heart.
Hepatic selenium analysis was within normal limits for a yearling
llama.
-
- Contributor's Diagnosis and Comments: Multifocal,
moderate, acute necrotizing myocarditis and myositis with intralesional
protozoal zoites (Toxoplasma gondii).
-
- Hallmarks of systemic toxoplasma infection include interstitial
pneumonitis, focal hepatic necrosis, myocarditis, lymphadenitis,
and nonsuppurative meningoencephalitis. Systemic toxoplasmosis
has been reported in most species of domestic animals, but is
most common in immunologically immature neonates and immunosuppressed
hosts, such as dogs with canine distemper virus infection and
human patients with acquired immunodeficiency syndrome. Serologic
surveys indicate that up to one third of llamas have been exposed
to this parasite, but with the exception of recent reports describing
rising toxoplasma titers in llama abortions, there are few reports
of disseminated disease in camelids.
- Protozoal organisms consistent with Toxoplasma gondii were
readily distinguishable among foci of necrosis and nonsuppurative
inflammation in the heart and diaphragm of this young llama.
Organisms were also identified in the gastric smooth muscle underlying
the C-3 compartment ulcerations, and in association with multifocal
necrotizing adrenalitis, thyroiditis, and encephalitis as well.
Mild multifocal hepatic necrosis and hepatitis, mild nonsuppurative
interstitial nephritis, and focal pulmonary interstitial necrosis
were also observed in this animal, although organisms were not
identified in these lesions. Concurrent evidence of lymphoid
depletion suggested juvenile llama immunodeficiency syndrome
as a probable factor underlying the development of disseminated
infection in this animal.
-
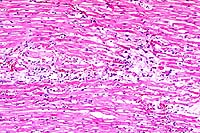 10x
obj
10x
obj
- Case14-4. Heart. There are multiple coalescing foci
of myocardial cell degeneration, necrosis, edema, and an inflammatory
cell infiltrate.
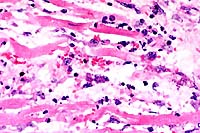 40x
obj
40x
obj
- Case14-4. Heart. This focus of necrosis has loss of
cross striations, hyalinization, and fragmentation of cardiomyocytes,
edema, with karyorrhectic nuclei and scattered macrophages. There
are two clusters of 2-3u diameter protozoa.
-
- AFIP Diagnosis: Heart and diaphragm: Myositis, necrotizing,
lymphohistiocytic, subacute, multifocal, moderate, with intracellular
and extracellular protozoa, llama (Llama glama), camelid.
-
- Conference Note: Conference participants identified
moderate numbers of protozoal organisms associated with foci
of necrosis and inflammation in the heart and diaphragm. Because
the tachyzoites of Toxoplasma gondii and Neospora caninum are
morphologically indistinguishable in routine hematoxylin and
eosin stained sections, unstained tissue sections were submitted
to Dr. J.P. Dubey of the Agricultural Research Center of the
United States Department of Agriculture for immunohistochemical
studies. The protozoal organisms in the heart and diaphragm are
immunohistochemically positive for Toxoplasma gondii.
-
- Toxoplasma gondii is a coccidian protozoal parasite of the
phylum Apicomplexa and is characterized by small (4-6mm long),
crescentic, tachyzoites. The protozoan may form tissue cysts
in infected animals that are spherical to elongate, have a thin
0.5mm wall, measure between 10-100mm in diameter, and are found
in various tissues including muscle, liver, retina, and brain.
Toxoplasma gondii, unlike other protozoa with the exception of
Neospora caninum, has the ability to infect a wide range of homeothermic
hosts, and natural infection has been reported in birds, nonhuman
primates, rodents, insectivores, herbivores, carnivores, and
in humans. Domestic and wild felids are the only definitive hosts,
while both felids and nonfelids serve as intermediate hosts.
While documented reports of toxoplasmosis in llamas are scarce,
it is not surprising that this species is susceptible to infection.
-
- Transmission of toxoplasma protozoa can occur to intermediate
hosts by ingestion of oocysts in feline feces, ingestion of cysts
from the tissue of infected animals (meat), and transplacentally
via tachyzoites, especially in sheep and goats in which the organism
is an important cause of abortion. Upon ingestion of sporulated
oocysts, sporozoites excyst and multiply as tachyzoites in the
intestines and associated lymph nodes. The tachyzoites continue
to multiply, and eventually parasitemia develops, disseminating
the protozoa to various tissues where the organisms penetrate
a variety of cell types, including macrophages, fibroblasts,
and smooth muscle cells. Actively replicating tachyzoites are
found within a parasitophorous vacuole in infected cells. Necrosis
is a common feature of disseminated disease and is due to continued
replication of the organisms, leading to cell death. Cell to
cell transmission may occur within an infected organ, and the
characteristic histologic findings are variably sized foci of
necrosis, nonsuppurative inflammation, and the presence of intracellular
tachyzoites in the vicinity of necrotic areas.
-
- In most instances of disease, immunity to toxoplasmosis develops
in a few days which reduces but does not terminate infection.
Immune animals develop a dormant form of disease characterized
by the formation of bradyzoite-filled cysts within 1-2 weeks
of initial infection. Functional cell-mediated immunity is important
for inducing cyst formation and eliminating tachyzoites from
the circulation and visceral organs. Lymphoid depletion, immunodeficiency,
and loss of cell-mediated immunity may be the underlying cause
for the fulminant case of toxoplasmosis in this llama.
-
- Differential diagnosis briefly discussed by conference participants
included other protozoa, such as Neospora caninum, Leishmania
sp., Trypanosoma, and Sarcocystis and the yeast forms of Histoplasma
capsulatum. Thus far, the cysts of Neospora caninum have only
been identified in tissues of the central nervous system of infected
animals and are characterized by thick, 1-4mm, cyst walls; identification
of protozoal cysts within tissues other than the central nervous
system suggests infection with T. gondii. Leishmania and Trypanosoma
are morphologically characterized by the presence of a kinetoplast
perpendicular and parallel to the nucleus, respectively; tachyzoites
of T. gondii lack kinetoplasts. Sarcocystis have merozoites that
invade endothelial cysts, and the tissue cysts, primarily found
in the heart and skeletal muscle of wild and domestic ruminants,
may become so large as to be seen by the unaided eye. Histoplasma
capsulatum was included in the differential diagnosis based on
size and morphology of the yeasts in tissues. In histoplasmosis,
the organisms incite histiocytic and/or granulomatous inflammation
and stain with Grocott's methenamine silver (GMS) and other fungal
stains.
-
- Contributor: North Dakota State University, Veterinary
Diagnostic Laboratory, Van Es Hall, Fargo, ND 58105.
-
- References:
- 1. Cheney JM, Allen GT: Parasitism in llamas. Vet Clin N
Amer Food Anim Pract 5:217-225, 1989.
- 2. Barker IK, Van Dreumel AA, Palmer N: The alimentary system.
In: Pathology of Domestic Animals, Jubb, Kennedy, Palmer eds.,
4th ed., vol. 1, pp. 308-310, Academic Press, San Diego, 1993.
- 3. Hutchinson JM, Garry F: Update on llama medicine: Ill
thrift and juvenile llama immunodeficiency syndrome. Vet Clin
N Amer Food Anim Pract 10:331-343, 1994.
- 4. Jones TC, Hunt RD, King NW: Diseases caused by protozoa.
In: Veterinary Pathology, 6th ed., pp. 555-561, Williams and
Wilkins, Baltimore, 1997.
- 5. Gardiner CH, Fayer R, Dubey JP: Apicomplexa. In: An Atlas
of Protozoal Parasites in Animal Tissues, 2nd ed., pp. 53-60,
Armed Forces Institute of Pathology, Washington DC, 1998.
-
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
Return to WSC Case Menu
 10x
obj
10x
obj
 40x
obj
40x
obj
 20x
obj
20x
obj
 10x
obj
10x
obj
 10x
obj, Reticulin stain
10x
obj, Reticulin stain
 40x
obj, Reticulin stain
40x
obj, Reticulin stain
 10x
obj, Trichrome stain
10x
obj, Trichrome stain
 10x
obj
10x
obj
 40x
obj
40x
obj