Results
AFIP Wednesday Slide Conference - No. 12
10 December 1997
-
- Conference Moderator: MAJ Mark Mense
Diplomate, ACVP
Division of Pathology
Walter Reed Army Institute of Research
Washington, D.C. 20307-5100
-
- Return to WSC Case Menu.
Case I - W751-97 (AFIP 2597489)
-
- Signalment: Adult, male, wild rabbit.
-
- History: The animal was found dead adjacent to the
clinical staff offices. Deaths in feral rabbits in the vicinity
of the Clinical Center had been noted several days previous to
this animal being found.
-
- Gross Pathology: There were no external lesions. The
cadaver appeared fresh and internal lesions were limited to the
lungs which were diffusely edematous and contained multifocal
hemorrhages, and the spleen which was enlarged approximately
twice normal size and was dark. The liver was enlarged with an
accentuated pale lobular pattern.
-
- Laboratory Results: Immunohistochemistry on sections
of liver was positive for rabbit calicivirus.
-
- Contributor's Diagnoses and Comments:
- 1. Hepatitis, acute, zonal, periportal to midzonal, necrotizing,
severe.
- 2. Splenitis, acute, necrotizing, massive, with hemorrhage
and thrombosis.
- 3. Lung, intravascular fibrinous thrombosis, multifocal,
acute with minimal interstitial changes, rabbit calicivirus (rabbit
hemorrhagic disease).
-
- This case is typical for the disease in susceptible adult
rabbits, and is almost uniformly fatal. Many animals are found
dead without premonitory signs, and those found alive rapidly
become comatose and die quietly. Blood-stained frothy fluid may
exude from external nares, and the lungs are edematous. Typically,
there is complete destruction of the sinusoidal architecture
of the liver and spleen, and replacement by fibrinous coagulum
containing erythrocytes. Destruction of white pulp may occur
as in this case, but is not uniformly present and may be secondary
to stress rather than a direct effect. Acute coagulation necrosis
in the liver is typically periportal, extending to midzonal areas
as in this case, and is usually accompanied by a minimal inflammatory
response.
- Viral antigen can be localized to areas of splenic and hepatic
necrosis using immunohistochemical methods, and the virus appears
to specifically target sinusoidal lining cells in these organs.
Although thrombi are often found in the lungs and also occasionally
in other organs, these are not associated with localization of
viral antigen in affected vessels by immunohistochemistry, although
viral antigens can be recovered from tonsils, lymph nodes and
kidneys using more sensitive methods such as RT-PCR. Intravascular
thrombosis is thought to cause rapid death, but histopathologic
evidence of DIC may wane if necropsies are delayed for long after
death. DIC is thought to develop largely because of the severe
necrosis of the liver.
- Rabbit hemorrhagic disease caused devastating losses of farmed
rabbits in Europe and Asia beginning in 1984. Baby rabbits do
not support viral replication to the degree that is found in
adults, and younger rabbits (<4 weeks) do not develop overt
signs after calicivirus infection. After extensive research,
the virus was released into Australia as a biologic method for
control of feral rabbits and although marked reduction in rabbit
numbers has occurred in some areas as a result, in other areas
rabbit numbers appear to have returned to pre-release levels.
Reasons for this remain unclear. Overseas, a non-pathogenic strain
of rabbit calicivirus has been identified and infection with
this strain may protect against the pathogenic isolate. In addition,
antibody is protective and exposure of young kits to the virus
may allow the establishment of immunity. Possible spread of the
virus by fomites, birds and insect vectors is suspected, but
in the unusual circumstances in Australia, where upon release/escape
in late 1995, cases were recorded up to hundreds of miles away
within days, and deliberate spread by the farming community was
suspected.
- A related calicivirus, European brown hare syndrome virus,
produces a similar disease in hares, but the two diseases are
distinct entities and cross infection or protection do not occur.
These two viruses are unlike other members of Caliciviridae (San
Miguel sea lion virus and feline calicivirus) in that they appear
to have little genomic variation between isolates. An effective
vaccine is available for use in laboratory, commercial and companion
rabbits and vaccination of these rabbit stocks in Australia was
possible prior to release of the virus. The use of rabbit calicivirus
as a biologic method of controlling a massive population of feral
rabbits precipitated intense debate. Because the virus kills
rabbits so quickly and moribund animals generally die quietly,
objections based on animal ethical grounds were largely dismissed.
Controversy remains as to whether the depletion of feral rabbit
numbers will have a beneficial effect on threatened native wildlife
species, because recovery of degraded habitats may be offset
by greater predation of these species by feral cats and foxes
that normally rely on rabbits as their major food source.
-
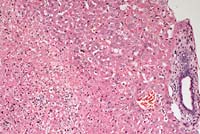
- Case 12-1. Liver. Acute necrosis with intact parenchyma.
10X
- AFIP Diagnoses:
- 1. Liver: Hepatitis, necrotizing, acute, periportal to midzonal,
diffuse, moderate, rabbit, lagomorph.
- 2. Lung: Fibrin thrombi, multifocal, with mild acute interstitial
pneumonia.
- 3. Lung, blood vessels: Medial hypertrophy, multifocal, moderate.
- 4. Spleen: Congestion, hemorrhage, and fibrin deposition,
acute, diffuse, severe, with lymphoid necrosis.
-
- Conference Note: Rabbit hemorrhagic disease (RHD)
virus affects rabbits of the species Oryctolagus cuniculus; no
other rabbit species have been shown to be susceptible.9 The
incubation period is 16-48 hours. Originally, reports from China
identified peracute, acute, and subacute forms of the disease.
Subsequent descriptions in both natural and experimental infections
have been consistent with a peracute form, in which rabbits die
suddenly with few or no clinical signs, and an acute form, which
is seen in areas where RHD is established and in which rabbits
exhibit clinical signs before death.
- RHD virus is spread by several routes and vectors. The virus
is present in excretory products, and enters susceptible hosts
usually by the oral or respiratory routes. The virus is stable
in the environment, and can be spread by fomites such as bedding
material or feedstuffs, or carried short distances by insects.
Foxes and polecats have been shown to seroconvert after ingestion
of the virus, though their role in transmission of the disease
is unclear. Rabbit products, including pelts and meat, serve
as potential sources of spread. An RHD epidemic in Mexico was
linked circumstantially to frozen rabbit carcasses imported illegally
through another country, though direct confirmation of disease
transfer from infected meat products was never demonstrated.9
- Comparatively, RHD resembles certain viral hemorrhagic diseases
of humans and nonhuman primates, including Lassa fever, simian
hemorrhagic fever, and Ebola and Marburg viral infections. The
pathogenic mechanisms of DIC in these diseases are not completely
understood.
-
- Contributor: School of Veterinary Science, The University
of Melbourne, 250 Princes Highway, Werribee, Victoria 3030, Australia
-
- References:
- 1. Brander P, Boujon CE, Bestetti GE: Infectious hemorrhagic
disease of rabbits in the Bern Pathology Institute (1988-1990):
season and regional distribution and histopathological findings.
Schweiz-Arch-Tierheilkd. 134(8):383-389, 1992.
- 2. Capucci L, Fusi P, Lavazza A, Pacciarini ML, Rossi C:
Detection and preliminary characterization of a new rabbit calicivirus
related to rabbit hemorrhagic disease virus but nonpathogenic.
J Virol 70(12):8614-8623, 1996.
- 3. Chasey D, Lucas M, Westcott D, Williams M: European brown
hare syndrome in the U.K.; a calicivirus related to but distinct
from that of viral hemorrhagic disease in rabbits. Arch Virol
124(3-4):363-370, 1992.
- 4. Fuchs A, Weissenbock H: Comparative histopathological
study of rabbit hemorrhagic disease (RHD) and European brown
hare syndrome (EBHS). J Comp Pathol 107(1):103-113, Jul 1992.
- 5. Guittre C, Ruvoen-Clouet N, Barraud L, Cherel Y, Baginski
I, Prave M, Ganiere JP, Trepo C, Cova L: Early stages of rabbit
hemorrhagic disease virus infection monitored by polymerase chain
reaction. Zentralbl-Veterinarmed-B 43(2):109-118, Apr 1996.
- 6. Ueda K, Park JH, Ochiai K, Itakura C: Disseminated intravascular
coagulation (DIC) in rabbit hemorrhagic disease. Jpn-J-Vet-Res
40(4):133-141, Dec 1992.
- 7. Wirblich C, Thiel HJ, Meyers G: Genetic map of the calicivirus
rabbit hemorrhagic disease virus as deduced from in vitro translation
studies. J Virol 70(11):7974-7983, Nov 1996.
- 8. Park JH, Lee Y-S, Itakura C: Pathogenesis of acute necrotic
hepatitis in rabbit hemorrhagic disease. Lab Anim Sci 45(4):445-449,
1995.
- 9. Chasey D: Rabbit haemorrhagic disease: the new scourge
of Oryctolagus cuniculus. Laboratory Animals 31:33-44, 1997.
-
- International Veterinary Pathology Slide Bank:
Laser disc frame #14546
-
Case II - 95051363 (AFIP 2596338)
-
- Signalment: 23-day-old, female, Quarter Horse.
-
- History: This foal appeared depressed and icteric
at 6:30 a.m. and was found dead at 12:30 p.m.
-
- Gross Pathology: The foal was moderately icteric.
The stomach had multiple small foci of ulceration in the non-glandular
region. The liver was moderately enlarged with rounded edges.
On cut surface, the liver bulged, the lobular pattern was accentuated,
and the liver was yellow.
-
- Laboratory Results: Bacteriology results: Only a few
contaminating bacteria (E. coli and Enterobacter sp.) were isolated
from the liver.
-
- Contributor's Diagnosis and Comments: Liver, hepatitis,
suppurative, with necrosis, multifocal, coalescing, severe, compatible
with Tyzzer's disease of neonatal foals.
- Intracellular bacteria compatible with Clostridium piliforme
(Bacillus piliformis) were demonstrated in histopathological
sections. Lesions and organisms are compatible with Tyzzer's
disease in foals.
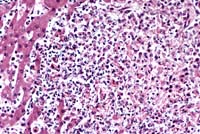
- Case 12-2a. Liver. Locally extensive hepatic necrosis
with an influx of neutrophils. 20X
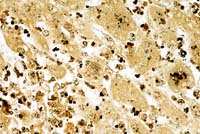
- Case 12-2b. Liver. Note the intracellular cluster
of long slender bacilli (Clostridium piliformis). Warthin-Starry
40X
AFIP Diagnoses:
- 1. Liver: Hepatitis, necrotizing, neutrophilic, acute, multifocal
to coalescing, random, severe, Quarter Horse, equine.
- 2. Liver: Cholestasis, canalicular, multifocal, moderate.
-
- Conference Note: Tyzzer's disease is an acute, highly
fatal, epizootic enterohepatic disease of neonatal or weanling
animals. It has been reported in numerous animal species, including
horses, cattle, mice, rats, hamsters, guinea pigs, rabbits, foxes,
and coyotes. The causative agent, Clostridium piliforme, is a
gram-negative, spore-forming, motile, obligate intracellular
bacterium with peritrichous flagella. The vegetative form causes
the disease state, and appears as bundles or "haystacks"
within its target cells, i.e. enterocytes and hepatocytes. Visualization
of the bacteria in histologic section is enhanced with silver
stains such as the Warthin-Starry procedure.
- In horses, Tyzzer's disease usually affects one- to six-week-old
foals. Clinical signs include weakness, rapid respiratory rate
and pulse, cold extremities, icterus, blindness, and severe depression
before death, which occurs within 2 to 48 hours. Diarrhea may
also be observed. Outbreaks of the disease are sporadic, typically
affecting very few animals in a herd. This suggests that the
disease is not highly contagious, and may be limited to immunocompromised
foals.1
- The pathogenesis of equine Tyzzer's disease is incompletely
understood. Clostridium piliforme is transmitted by the fecal-oral
route. The initial site of infection is the intestinal epithelium,
from which the organism is transported hematogenously to the
liver. In rodents, transplacental transmission also occurs, but
this has not been demonstrated in the horse.
- Gross lesions include icterus, hepatomegaly, and multifocal
1- to 5-mm white foci scattered throughout the hepatic parenchyma.
In guinea pigs, mesenteric and colonic lymph nodes are swollen,
and the cecum and colon are reddened, thickened, distended, and
contain gray pinpoint necrotic foci. Similar lesions can be found
in hamsters, but in this species the most striking gross lesion
reported is multiple white elevated nodules in the heart, which
are necrotic foci with massive infiltrates of large macrophages,
neutrophils, plasma cells, lymphocytes, and fibroblasts interspersed
with the organisms.
-
- Contributor: Department of Anatomy, Pathology and
Pharmacology, 250 Veterinary Medical Bldg., Oklahoma State University,
Stillwater, OK 74078-2007.
-
- References:
- 1. Hook RR, Riley LK, Franklin CL, Besch-Williford CL: Seroanalysis
of Tyzzer's disease in horses: implications that multiple strains
can infect Equidae. Equine Vet J 1995 Jan;27(1):8-12.
- 2. Franklin CL, Motzel SL, Besch-Williford CL, Hook RR Jr,
Riley LK: Tyzzer's infection: host specificity of Clostridium
piliforme isolates. Lab Anim Sci 1994 Dec;44(6):568-72.
- 3. Humber KA, Sweeney RW, Saik JE, Hansen TO, Morris CF:
Clinical and clinicopathologic findings in two foals infected
with Bacillus piliformis. J Am Vet Med Assoc 1988 Dec 1;193(11):1425-8.
- 4. Frisk CS: Bacterial and Mycotic Diseases. In: Laboratory
Hamsters, Van Hoosier GL, McPherson CW, editors, Academic Press,
Inc., pp. 121-125, 1987.
-
- International Veterinary Pathology Slide Bank:
Laser disc frame #2456, 2934, 4195-9, 5406, 5463, 9305, 10173-4,
10927, 17147-50.
-
Case III - 97-5457 S10 (AFIP 2594500)
-
- Signalment: 18-year-old, male, castrated, Arabian
horse.
-
- History: Neurologic horse with grade 4 ataxia.
-
- Gross Pathology: In the sacral spinal canal there
was a thick, fibrous, extradural mass which formed a collar around
the sacral cord and filum terminale.
-
- Contributor's Diagnosis and Comments: Cauda equina
neuritis.
- The extradural mass seen grossly resulted from severe, diffuse,
granulomatous cauda equina neuritis where the inflammatory reaction
had become confluent. Individual nerve bundles were inflamed
and some were completely necrotic. An intense inflammatory response
was present in virtually all extradural nerve bundles, and fibrosis
and multinucleated giant cell formation added to its severity.
-
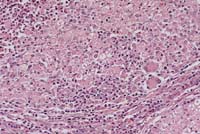
- Case 12-3. Cauda equina. The nerve bundle is replaced
by macrophages, multinucleated giant cells and a mass of neutrophils
in a necrotic area to the top left corner. The lower right shows
a mostly intact smaller nerve. 20X
AFIP Diagnosis: Cauda equina with adjacent adipose tissue:
Neuritis and ganglioneuritis, granulomatous, diffuse, severe,
with moderate chronic steatitis, Arabian horse, equine.
-
- Conference Note: Cauda equina syndrome is an uncommon
condition in horses in which the sacral and coccygeal nerves,
and occasionally cranial nerves V and VII, are chronically inflamed.
Resulting clinical signs include paralysis of the tail, rectum,
and bladder; anesthesia in the sacral dermatomes with a surrounding
zone of hyperesthesia; gluteal muscle atrophy; and hind limb
ataxia and weakness. In horses with cranial nerve involvement,
facial paralysis, head tilt, and masticatory muscle atrophy are
also seen.
- Marked granulomatous inflammation and proliferation of the
epineurial and perineurial sheaths are the major histopathologic
changes in the extradural nerve roots and spinal ganglia. Intradural
roots are usually less severely affected. Though some nerve fascicles
remain intact, many contain dense infiltrates of lymphocytes,
plasma cells, and macrophages extending into the fascicle interior.
Secondary to interruption of axons, retrograde chromatolysis
develops in the somatic motor neurons of the affected spinal
segments. Destruction of sensory neurons in the dorsal roots
and ganglia leads to orthograde nerve fiber degeneration in the
spinal dorsal funiculus.6
- The exact etiopathogenesis is unknown. Several studies have
demonstrated similarities between this condition and both the
Guillain-Barré syndrome in man and experimental allergic
neuritis (EAN) in rats. Kadlubowski and Ingram5 showed that affected
horses have circulating antibodies to P2 myelin protein, a neuritogenic
myelin antigen that on injection causes EAN. It is unclear whether
these circulating antibodies play a primary role in demyelination
or represent a consequence of antigen released in the course
of myelin destruction.6 In another study4, equine adenovirus
1 was isolated from cauda equina nervous tissue in 2 out of 3
horses with cauda equina syndrome. In 6 normal horses of similar
age, no viral agents were isolated. Though these findings suggest
a causal linkage, it is also plausible that the adenovirus infection
was established as an avirulent opportunistic infection in the
period of stress.
-
- Contributor: Oregon State University, College of Veterinary
Medicine, P.O. Box 429, Corvallis, OR 97339
-
- References:
- 1. Wright JA, Fordyce P, Edington N: Neuritis of the cauda
equina in the horse. J Comp Path 97:667-675, 1987.
- 2. Jubb KVF, Haxtable CR: The nervous system. In: Pathology
of Domestic Animals. Jubb KVF, Kennedy PC, and Palmer N., eds.,
Academic Press, San Diego, 4th ed., Vol. 1, p. 428, 1993.
- 3. Fordyce PS, Edington N, Bridges GC, Wright JA, Edwards
GB: Use of an ELISA in the differential diagnosis of cauda equina
neuritis and other equine neuropathies. Equine Veterinary Journal
19(1):55-59, 1987.
- 4. Edington N, Wright JA, Patel JR, Edwards GB, Griffiths
L: Equine adenovirus 1 isolated from cauda equina neuritis. Res
Vet Sci 37:252-254, 1984.
- 5. Kadlubowski M, Ingram PL: Circulating antibodies to the
neuritogenic myelin protein, P2, in neuritis of the cauda equina
of the horse. Nature 293:299-300, 24 September 1981.
- 6. Summers BA, Cummings JF, de Lahunta A: Veterinary Neuropathology,
Mosby-Year Book, Inc., St. Louis, MO, pp. 432-434, 1995.
International Veterinary Pathology Slide Bank:
Laser disc frame #1588-9, 1676, 2104, 6980, 10539-43, 14179-81,
17068-72, 17115-8.
-
Case IV - 1648 or 1649 (AFIP 2600782)
- Signalment: 4-month-old, 65 kg, male, castrated, German
Landrace pig.
-
- History: To study the experimental infection with
Oesophagostomum dentatum, pigs were orally inoculated with a
single dose of 50,000 infective larvae of Oesophagostomum dentatum.
None of the animals developed clinical signs of diarrhea. The
tissues are from an animal which was euthanized at day 7 post-inoculation.
-
- Gross Pathology: Macroscopic lesions were predominantly
found in the cecum and proximal colon. The mucosa was severely
edematous and hyperemic. Numerous red and/or white nodules (1-5
mm in diameter) were visible in the mucosa.
-
- Contributor's Diagnosis and Comments: Moderate granulomatous
typhlocolitis with nematode larvae.
-
- Etiology: Oesophagostomum dentatum.
- Sections of nematode larvae are present in the lamina propria
- sometimes extending to the submucosa. Nematode larvae are surrounded
by an amorphous eosinophilic capsule which occasionally contains
neutrophils and macrophages, by a thin layer of neutrophils and
macrophages and by a thick layer of lymphocytes. Eosinophils
and mast cells are quite numerous in some parasitic nodules.
They are predominantly located in the periphery of the nodules
and in the submucosa. Tangential section through parasitic nodules
revealing only mononuclear infiltrates in the lamina propria
and submucosa are frequent. Some of them have central areas of
necrosis. The submucosa is mildly to moderately infiltrated by
lymphocytes, macrophages and eosinophils. These infiltrates extend
in some slides into the tunica muscularis and serosa.
- Sections through mucosa-associated lymphoid nodules are present
in some slides. They can be distinguished from parasitic granulomas
by the presence of lymphoid follicles which contain tingible
body macrophages and are surrounded by a collagenous capsule.
- Oesophagostomum dentatum belongs to the family Stongylidae.
It is a large intestinal nematode of pigs. Three species of Oesophagostomum
occur in pigs: O. dentatum, O. quadrispinulatum and O. brevicaudum.
Pigs become infected by oral uptake of infectious larvae. After
24 hours, these larvae penetrate the mucosa of the cecum and
proximal large intestine. They induce the formation of parasitic
granulomas in the lamina propria and submucosa. In these granulomas
they mature and return as 4th stage larvae to the intestinal
lumen between days 7 and 14 post-infection (pi). At day 14 pi,
only mild multifocal infiltrates of macrophages, lymphocytes
and eosinophils remain in the mucosa. In the intestinal lumen
the 4th stage larvae develop to adults (females: 11-15 mm, males:
8-10 mm) and start shedding eggs.
- O. dentatum has a high incidence especially in breeding pigs.
Severe infections may result in necrotizing enteritis. In most
herds, the infection is not recognized since it is frequently
subclinical. It causes, however, reduced weight gain, reduced
litter size, and may complicate other infections. Thus, it is
of economic importance. Infection with O. dentatum in pigs induces
inflammatory reactions in the intestinal mucosa, but this does
not result in protective immunity.
-
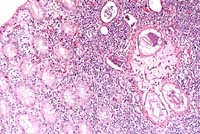
- Case 12-4. Colon. Nematode larvae (Oesophagostomum
dentatus) in the mucosa is surrounded by mononuclear leukocytes
and bordered by several crypt abscesses. 10X
- AFIP Diagnosis: Colon: Colitis, subacute and eosinophilic,
multifocal, moderate, with
- nematode larvae, German Landrace, porcine, etiology consistent
with Oesophagostomum spp.
-
- Conference Note: Oesophagostomum infection is found
world-wide. In addition to pigs, Oesophagostomum spp. are important
intestinal parasites of various ruminants and primates, including
man. In cattle, O. radiatum infection can result in severe weight
loss, anorexia, anemia and diarrhea. O. columbianum and O. venulosum
affect sheep, goats and certain wild ruminants. These species
have a similar life cycle to those affecting pigs, but the larvae
of each species infect different sections of the intestine. Adult
worms of all species are found in the cecal and colonic lumina.
-
- Oesophagostomiasis has been reported to be the most frequent
and pathogenic helminth of laboratory primates, and can represent
a serious threat to the health of colonies.2 Gross lesions include
dark nodules up to 8 mm in diameter within the submucosa or serosa
of the cecum and colon, and occasionally in the mesocolon. Incidences
of up to 70% in rhesus monkeys and 62% in cynomolgus monkeys
have been reported.
Contributor: Institut für Pathologie, Tierärztliche
Hochschule Hannover, Bünteweg 17, 30559 Hannover, Germany.
-
- References:
- 1. McCracken RM, Ross JG: The histopathology of Oesophagostomum
dentatum infections in pigs. J Comp Pathol 80:619-623, 1970.
- 2. Stewart TB, Gasbarre LC: The veterinary importance of
nodular worms (Oesophagostomum spp.). Parasitol. Today 5:209-213,
1989.
- 3. Stockdale PHG: Necrotic enteritis of pigs caused by infection
with Oesophagostomum spp. Br Vet J 126:526-529, 1970.
- 4. Abbott DP, Majeed SK: A survey of parasitic lesions in
wild-caught, laboratory-maintained primates: (rhesus, cynomolgus,
and baboon). Vet Pathol 1984 Mar;21(2):198-207.
-
- International Veterinary Pathology Slide Bank:
Laser disc frame #5284, 13612
-
- Terrell W. Blanchard
Major, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: blanchard@email.afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
-
- Return to WSC Case Menu.