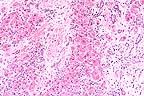
 Marked bridging portal and
centrilobular fibrosis and biliary hyperplasia separates remaining
hepatocytes and is suggestive of toxic hepatopathy in cattle.
(HE, 100X, 64K)
Marked bridging portal and
centrilobular fibrosis and biliary hyperplasia separates remaining
hepatocytes and is suggestive of toxic hepatopathy in cattle.
(HE, 100X, 64K)
Signalment: 8-month-old Holstein steer.
History: This steer from a small farm died while being transported to a diagnostic laboratory. The owner said the animal had suddenly stopped eating and had become lethargic the day before. The only significant gross lesion was a pale and extremely firm liver.
Gross Pathology: See above.
Laboratory Results: N/A
Contributor's Diagnosis and Comments: Severe, diffuse, portal fibrosis of the liver, with bile duct hyperplasia and toxic hepatopathy due to pyrrolizidine alkaloid toxicity.
Widespread portal fibrosis, bile duct proliferation, and megalocytosis are characteristic lesions observed in the liver of cattle poisoned by plant-derived pyrollizidine alkaloids. These chemicals are found in a variety of plant families, particularly such genera as Senecio, Crotalaria, Heliotropium and Amsinckia. Groundsel (Senecio spp.) was identified in the hay that had been fed to this animal.
The pyrrolizidine alkaloids are metabolized to toxic electrophilic pyrroles, which cause alkylation reactions with amino acids and their derivatives. Dissociation of the alkylation products may result in the formation of new alkylating agents, which may cause cellular damage to persist after ingestion of the alkaloid has ceased.
The kinetics of conversion of aklyloids to pyrroles, and the chemical nature of these products, varies not only with species, but also with animal age and sex, and with the metabolic rate of the target cells; hence the difficulty in providing a prognosis of individual animals following consumption of the toxin. The megalocytosis is due to an antimitotic effect, but the mechanisms responsible for this change, as well as the fibrosis and bile duct proliferation, are not well understood.
In addition to the hepatotoxic effect, these alkaloids have been found to result in elevated hepatic copper levels and diminished plasma and liver vitamin A levels in some animals.
In Oregon (USA), this toxic hepatopathy was common in cattle, due to the abundance of tansy ragwort (Senecio jacobaea) in pastures and hay. However, in recent years, exposure of farm animals to this plant has been dramatically reduced through biological control. Controlled release of the cinnabar moth, ragwort flea beetle, and ragwort seed fly, whose larva feed upon the tansy plants, has significantly reduced economic losses associated with pyrrolizidine toxicity.
AFIP Diagnosis: Liver: Fibrosis, portal and bridging, diffuse, severe, with diffuse perivenous fibrosis and obliteration, biliary hyperplasia and mild megalocytosis, Holstein, bovine.
Conference Note: Pyrrolizidine alkaloids have been found in various species of plants widely distributed in the world. In addition to those listed by the contributor, the genera Cynoglossum, Echium, and Trichodesma have also been known to cause disease.
The most characteristic effect of toxic pyrroles is the induction of nuclear and cytoplasmic gigantism (megalocytosis). This effect is most likely due to an antimitotic effect with continued DNA synthesis. Continued nucleoprotein synthesis, coupled with mitotic inhibition, probably accounts for the great increase in size of the nucleus and cytoplasm. Megalocytic cells can range up to 20 times normal. Other alkylating agents such as nitrosamine and aflatoxins can also result in megalocytosis. Concurrent with the development of megalocytosis, there is fibroplasia and proliferation of the bile ducts. This fibroplasia is generally minimal in sheep, moderate in horses and may be marked in cattle.
Acute poisoning by the pyrrolizidine alkaloids is uncommon and results in periacinar necrosis and endothelial damage to the hepatic venules and small hepatic veins. This form of toxicosis is not clearly distinguishable from a variety of other hepatotoxins.
In cattle, chronic pyrollizidine alkaloidosis produces pronounced hepatic bridging portal fibrosis which infiltrates along the sinusoids to dissect lobules, separate individual cells, and link the walls of efferent veins. This form of fibrosis has been termed "veno-occlusive disease" and was originally described in "bush tea poisoning" in man.
In sheep, long term consumption of pyrrolizidine containing plants may lead to elevated levels of liver copper followed by the hemolytic crisis of copper toxicity. In pigs, pyrrolizidine alkalosis primarily manifests as pneumonia and renal insufficiency. Pulmonary emphysema is a characteristic finding in pigs and horses. The pulmonary toxicity of pyrrolizidine alkaloids in rats is well recognized. The primary site of injury in this species appears to be the alveolar septa. The lesions include severe vascular engorgement and edema, and diffuse fibrosis of alveolar and interlobular septa with patchy epithelialization.
Contributor: Veterinary Diagnostic Laboratory, Oregon State University, P.O. Box 429, Corvallis, OR 97339-0429.
References:
1. Kelly, WR. The liver and biliary system. In: Pathology of Domestic Animals, 4th ed., KVF Jubb, PG Kennedy, and N Palmer, eds., Academic Press, San Diego, CA; 1993;pp. 392-395.
2. Cheeke, PR. Copper, Vitamin A, and pyrollizidine alkaloid interactions in livestock and laboratory animals. In: Poisonous plants: Proceedings of the Third International Symposium; LF James et al (eds); Iowa State University Press, Ames, IA; 1992; pp.175-180.
3. Kingsbury, JM. Poisonous plants of the United States and Canada; Prentis Hall, Inc., Englwood Cliffs, NJ; 1964; pp. 425-435.
4. Blood, DC; Radostits, OM: Veterinary Medicine, 7th ed. Bailliere Tindall, 1989; pp. 1340-41.
International Veterinary Pathology Slide Bank: Laser disc frame #13157, 21280, 21281, 24611, 6801, 6802, 9316, 9332.
Signalment: Female broiler chickens, 5 weeks of age.
History: The submitting grower farm had experienced increased condemnation rates. Many of the birds in previous flocks had been diagnosed with E. coli septicemia at about 5 weeks of age.
Gross Pathology: Eight birds were necropsied. Lesions included marked increase in light yellow, thin fluid within the abdominal cavity. Small multifocal mucosal ulcerations, measuring approximately 2 mm in diameter, were noted within the gizzard of some birds. Moderate amounts of thick, yellow to light tan exudative material was noted within the lumen of the trachea and adherent to the thoracic and abdominal air sacs and the pleura of the lung. The bursa of Fabricius of each bird was moderately decreased in size.
Laboratory Results: 1. Bacteriology: a. Air sacs (abdominal and thoracic) - Moderate growth of E. coli (non-lactose fermenting). 2. Virus isolation: Positive for infectious bronchitis virus (IBV), untypable isolate.
Contributor's Diagnosis and Comments: 1. Moderate acute to subacute protozoan parasitic cloacal bursitis (Cryptosporidium sp.) 2. Mild lymphoid depletion - bursa of Fabricius. 3. Mild acute lymphoid follicular necrosis - bursa of Fabricius.
Multiple sections of the bursa of Fabricius were submitted. Moderate diffuse intraepithelial and superficial subepithelial accumulations of heterophils were evident. Mild acute lymphoid necrosis was noted within many of the bursal lymphoid follicles. Occasional intraepithelial cystic structures, many containing amorphous basophilic material and cellular debris, were also noted within the sections. Mild lymphoid depletion was also evident. Numerous round to ovoid basophilic structures measuring approximately 4-6 m, in diameter were noted adherent to the mucosal surfaces of the tissues.
Cryptosporidiosis is produced by coccidian parasites of the genus Cryptosporidium. Natural infections of the epithelial cells of the respiratory, genitourinary, and gastrointestinal tracts by this organism have been reported in a variety of avian species, including chickens, turkeys and quail. The organisms have also been reported in the urinary tract of a finch and jungle fowl and the esophagus and proventriculus of selected avian species. Two species (C. baileyi and C. meleagridis) appear to infect both chickens and turkeys. A third, unnamed species has been implicated in infections in quail. In chickens and turkeys, C. baileyi is believed to be the etiologic agent in intestinal (cloacal and bursa of Fabricius) and respiratory cryptosporidiosis. C. meleagridis is believed to be responsible for the small intestinal form of the disease in turkeys. Inhalation/aspiration or ingestion of oocysts of C. baileyi are responsible for the respiratory form and intestinal forms of cryptosporidiosis, respectively. Respiratory signs induced experimentally in young broiler chickens consist of sneezing and coughing with extension of the head noted in some cases. Lesions noted in the experimentally produced respiratory form of the disease include airsacculitis and pneumonia. Intestinal (cloacal and bursa of Fabricius) cryptosporidiosis can produce histologic lesions, but does not produce gross lesions or overt signs of disease in the chicken. Some reports suggest effects on broiler performance. The interaction of C. baileyi with other pathogens of the respiratory system can predispose birds to secondary invasion by E. coli and infectious bronchitis virus (IBV) can also increase the severity of respiratory disease in chickens induced by C. baileyi.
AFIP Diagnosis: Bursa of Fabricius: Bursitis, acute, diffuse, mild, with surface- associated protozoa, broiler chicken, avian, etiology consistent with Cryptosporidium sp.
Conference Note: The contributor diagnosed lymphoid atrophy and necrosis of the bursa. The conference participants were unable to differentiate these changes from normal involution of the bursa without age-matched controls.
Cryptosporidium is a small apicomplexan protozoan that infects birds, mammals, fish, amphibians, and reptiles, in addition to being a significant zoonotic agent, particularly in immunocompromised human beings. Respiratory infection is most significant in birds, whereas the disease in mammals is usually enteric.
The cryptosporidial organism attaches to the glycocalyx of the epithelial cell. The location is often described as intracellular but extracytoplasmic. The organism is surrounded by a membrane of host origin. At the interface between the parasite and the host epithelial cell is an attachment zone containing a specialized feeder organelle. Macrogametes are characterized ultrastructurally by the presence of dark staining polysaccharide granules.
In some cases, Cryptosporidium is considered a primary pathogen; however, in most situations it is considered to cause severe infections only in immunocompromised hosts. Studies in mice suggest that both CD4+ T cells and gamma interferon act synergistically to prevent initiation of Cryptosporidium infections, but may act through independent mechanisms to limit the extent or duration of infection.
Contributor: Animal Diagnostic Laboratory, Department of Veterinary Science, Pennsylvania State University, University Park, PA 16802.
References:
1. Current, W.L.: Cryptosporidiosis. In: Diseases of Poultry, ed, Calnek, B.W., H. John Barnes, C.W. Beard, W.M. Reid, H.W. Yoder, 9th., pp. 797-804, Iowa State University Press, Ames, Iowa, 1991.
2. Goodwin. M.A.: Cryptosporidiosis in birds - A review., Avian Pathology, 18:365-384, 1989.
3. Goodwin, M.A.: Esophageal and proventricular cryptosporidiosis in a chicken. Avian Diseases, 39:643-645, 1995.
4. Cheville NF: Pathogenic Protozoa. In: Ultrastructural Pathology: An Introduction to Interpretation, ed. Cheville NF, pp. 742-743. Iowa State University Press, Ames, IA, 1994.
5. Ungar BL, Kao TC, Burris JA, Finkelman FD: Cryptosporidium infection in an adult mouse model: Independent roles for IFN-gamma and CD4+ T lymphocytes in protective immunity. J of Immun 147:1014-1022, 1991.
6. Ritchie, BW; Harrison, GJ; Harrison, LR: Avian Medicine, pp. 1015-6, Wingers Publishing Inc., 1994.
International Veterinary Pathology Slide Bank: Laser disc frame #5224, 5225, 6170, 6171, 16272, 16273, 16274.
Signalment: 2-year-old neutered male domestic shorthair cat.
History: Two cats on the property had turned "yellow" and died, so when this cat became ill and developed icterus, it was taken to the veterinarian where the cat died.
Gross Pathology: Obese cat with large mass adjacent to the trachea, in the mediastinum and also involving pancreas,omentum and small intestine. Fluid present in abdominal and thoracic cavities. Cat was icteric.
Laboratory Results: Negative FeLV/FIV test by Elisa.
Contributor's Diagnosis and Comments: Disseminated mycobacteriosis - lung, liver, pancreas, lymph nodes. Mycobacterium avium was isolated on culture by the Public Health Department , Province of Alberta. Slides include "pancreatic" mass, intestine or omentum.
The cat did not appear debilitated but had generalized mycobacteriosis. Six cats were in the household; three died. The owner was a nurse. The cats came from a dairy farm but had lived in the city in the same location for at least 1 year.
AFIP Diagnosis: Mesentery: Mesenteritis, granulomatous and necrotizing, chronic, focally extensive, severe, with intrahistiocytic acid-fast bacilli, Domestic Shorthair, feline.
Conference Note: Mycobacterium avium and Mycobacterium intracellulare share many of the same properties making distinquishing them difficult. For this reason, the causative organisms are often referred to as M. avium-intracellulare complex or MAIC.
Dogs and cats are believed to be very resistant to infection with MAIC. These organisms are ubiquitous saprophytic opportunists and tend to cause disease in immunocompomised patients. Disseminated infection in cats has been rarely reported. In all cases, the cats tested negative for FeLV antigen and FIV antibody. However, an immune deficiency has been implicated. Siamese cats may have a more generalized predisposition to infection with intracellular organisms. Genetic susceptibility to mycobacteria has been identified in humans, rabbits, Basset hounds, and mice. In some families of inbred mice, genetic susceptibility to mycobacteria is associated with a single autosomal gene, Bcg, that shares a locus with a gene coding for resistance to two other intracellular pathogens.
In most mammals, MAIC infections are characterized by a diffuse granulomatous, inflammatory reaction characterized by large numbers of macrophages filled with numerous intracytoplasmic bacilli without necrosis, fibrosis, or calcification. Multinucleate giant cells of the Langhans type are often present. There is usually little lymphocytic response. Lesions are usually multifocal and coalescing or diffuse. Regional lymph nodes are typically involved and infection may become generalized via the hematogenous route. In this case, emboli of necrotic material and bacteria were noted within vascular structures.
The pathogenesis of mycobacterial infection is a complex and multifactoral process involving the organism and host immune response. Mycobacteria rely on thier ability to escape killing by macrophages and induce delayed type hypersensitivity. This has been attributed to several components of the mycobacterial cell wall. Antigen specific CD4+ T-cells are thought to confer resistance to most mycobacterial infections through the secretion of macrophage activating factors such as interferon-gamma.
Contributor: Central Veterinary Laboratory, 5645 199th Str. Langley B.C.V3A-1H9.
References:
1. Drolet, R.: Disseminated tuberculosis caused by Mycobacterium avium, JAVMA, 1986, 189(10):1336-7.
2. Kaufman, AC, et al: Treatment of localized Mycobacterium avium complex infection with clofazimine and doxycycline in a cat. JAVMA, 1995, 207(4):457-459.
3. Cotran RS, Kumar V, Robbins SL: Robbins, Pathologic Basis of Disease, 5th ed., WB Saunders, p. 324, 1994.
4. Hines, ME, et al: Mycobacterial infections of animals: pathology and pathogenesis, 1995, Lab An Sci, 1995, 45(4), 334-351.
5. Jordan, HL, et al: Disseminated Mycobacterium avium complex infection in three Siamese cats. JAVMA, 1994, 204(1): 90-93.
International Veterinary Pathology Slide Bank: Laser disc frame #5327, 9127, 21897.
Signalment: Six-day-old male crossbred lamb.
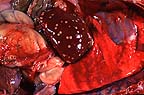 Multifocal areas of necrosis
in the liver of a six-day-old crossbred lamb (28K)
Multifocal areas of necrosis
in the liver of a six-day-old crossbred lamb (28K)
History: This animal was weak soon after it was born. No treatment was given.
Gross Pathology: The carcass was thin. The liver was enlarged and had multiple, well demarcated to coalescing, 2 to 10 mm diameter, yellowish foci with a caseous consistency throughout the parenchyma. On cut section, the wall of the rumen had a 5 x 7 cm thickened area with a yellowish-red and dry appearance and was very friable. Segments of the omentum were attached to the serosal surface of the rumen in the affected areas. No other gross lesion were observed.
Laboratory Results: Bacteriology: Liver isolates had the phenotypic, biological and enzymatic characteristics of Fusobacterium necrophorum subsp. necrophorum (Dr. Charles M. Scanlan, Department of Pathobiology, College of Veterinary Medicine, Texas A & M University).
Histopathology: The liver had multifocal to coalescing non-encapsulated areas of eosinophilic and amorphous material admixed with cellular debris (caseous necrosis) with minimal inflammatory reaction. There were numerous colonies of gram-negative filamentous bacteria and occasional cocci in the periphery of the necrotic areas. The adjacent sinusoids contained lymphocytes and neutrophils and sinusoidal cells were prominent. Occasionally (not in all slides), there were necrotic foci adjacent to veins (mainly central veins) and there was focal disruption of the vein wall with invasion of filamentous organisms into the vessel lumen and fibrin thrombi. Electron microscopy of the liver lesions demonstrated numerous, 1-2.5 m in length, rods with morphology characteristic of gram-negative bacteria and straight or blunt borders. The organisms were located both within hepatocytes and free in the interstitium.
There were also microscopic lesions in the rumen and heart (not submitted). The wall of the rumen in the grossly affected area had ulcerations and full thickness necrosis. The margins of the necrotic tissue contained numerous colonies of gram-negative filamentous bacilli as well as aggregates of gram-positive cocci and coccobacilli. Numerous degenerated leukocytes were at the periphery of the ulcer. There was fibrinoid necrosis of the vessels at the base of the ulcer with occasional fibrin thrombi and filamentous bacteria invading the walls. The left ventricle of the heart had multifocal areas of necrosis and suppuration with intralesional filamentous bacilli. There was multifocal fibrinosuppurative pleuritis and thymic lymphoid depletion.
Contributor's Diagnosis and Comments: Hepatitis, necrotizing, caseous, multifocal to focally extensive, with intralesional filamentous gram negative bacilli, vascular thrombosis. Rumenitis, necrotizing, caseous, focally extensive, transmural, with intralesional gram negative filamentous bacilli, fibrinoid vasculitis and thrombosis. Myocarditis, necrotizing, multifocal, with intralesional gram negative filamentous bacilli.
F. necrophorum is a common inhabitant of the environment of farm animals and the routes of infection in lambs are considered to be similar to those in cattle. Vascular drainage from the primary lesion (omphalitis or rumenitis) leads to localization in the liver. In the case reported here, the route of infection was believed to be ruminal with dissemination to the liver and other organs. This is supported by the lack of evidence of navel infection and the types of lesions found in the rumen and liver. The rumen had a severe inflammatory reaction associated with the presence of filamentous bacteria as well as vasculitis and thrombosis. Hepatic lesions had minimal inflammation suggesting more recent onset than in the rumen. Septicemic conditions due to Fusobacterium necrophorum are uncommon in domestic species but the presence of lesions in the heart are suggestive of it. The primary cause of the development of the ruminal lesions could not be determined but it has been considered that other infectious agents or feeding practices might predispose to this disease.
AFIP Diagnosis: Liver: Necrosis, coagulative, multifocal and focally extensive, perivascular and random, with necrotizing vasculitis and numerous extracellular filamentous bacteria, breed unspecified, sheep, ovine.
Conference Note: Infection by Fusobacterium necrophorum occurs in many species of farm animals. It is a normal inhabitant of the anaerobic ruminal environment. In most cases of extra-enteric disease, the organism is present as a secondary invader. Ruminal acidosis is a common predisposing factor for infections of forestomachs and liver. Other common diseases caused by Fusobacterium necrophorum include necrotic stomatitis, naval ill, pneumonia in calves, and foot rot in a variety of hoofstock.
The factors which contribute to the pathogenicity of Fusobacterium necrophorum include a potent endotoxin, a polysaccharide capsule, an exotoxin (leukocidin) and a hemolysin. It is postulated that the leukocidin allows the organism to withstand the phagocyte response and enables the infection to persist. In most instances, infection is minor and produces no clinical signs; with sufficient hepatic involvement, endotoxemia develops, causing an acute or chronic illness. Hematogenous spread from hepatic foci, including rupture into the caudal vena cava, may result in disseminated infection and rapid death.
Contributor: Animal Health Diagnostic Laboratory, P.O. Box 30076, Lansing, Michigan 48909-7516.
References:
1. Radostits OM, Blood DC, Gay CC: Diseases caused by Fusobacterium and Bacteriodes spp. In: Veterinary medicine: A textbook of the diseases of cattle, sheep, pigs, goats and horses. Baillier Tindall, 8th ed., pp. 865-881, 1994.
2. Scanlan CM, Berg JN: Comparative changes in a rat liver abscess model induced with three Fusobacterium necrophroum strains. Am J Vet Res 47:924-927, 1986.
3. Scanlan CM, Edwards JF: Bacteriologic and pathologic studies of hepatic lesions in sheep. Am J Vet Res 51:363-366, 1990.
4. Jubb, KVF; Kennedy PC; Palmer N: Pathology of Domestic Animals, 4th ed., Vol 2, pp. 48-49. 1994.
5. Scanlan CM, Hoyumpa AH, Ainsworth PC: A semiquantitative enzyme method for identifying Fusobacterium necrophorum biovars A and B. J Vet Diagn Invest. 4:86-87, 1992.
International Veterinary Pathology Slide Bank: Laser disc frame #449, 4081, 20437, 21130, 21131.
Lance Batey
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: batey@email.afip.osd.mil
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.