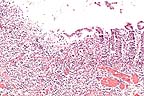
 Acute ulcerative colitis in
a foal treated with castor oil (HE, 200X, 50K).
Acute ulcerative colitis in
a foal treated with castor oil (HE, 200X, 50K).Signalment: 2-day-old Arabian colt.
 Acute ulcerative colitis in
a foal treated with castor oil (HE, 200X, 50K).
Acute ulcerative colitis in
a foal treated with castor oil (HE, 200X, 50K).
History: This foal developed mild colic between 12 and 24 hours after birth, with bloody diarrhea. The diarrhea and suspected failure of passive transfer of colostral antibodies were treated with mineral oil, castor oil, antibiotics, intravenous fluids, plasma and parenteral nutrition. There was a heart murmur, and on an echocardiogram there were vessels in the right atrium not associated with systemic venous circulation. Euthanasia was performed.
Gross Pathology: In the right atrium was a 5 x 3 x 3 cm mass composed of a thin wall of myocardium, divided into 3 compartments, opening into the right atrium beneath the crista terminalis and into a canal within the atrial septum that connected with an abnormal circumflex branch of the left coronary artery. There was mild interlobular pulmonary edema, with mild focal acute bronchopneumonia. In the middle of the length of the ventral large colon, there were several well defined 1-2 cm diameter raised green areas of mucosa.
Laboratory Results: Actinobacillus equuli was recovered from a clinical blood sample. Pooled cecal mucosa and small intestine contents were negative for Salmonella. These 2 areas of gut were cultured separately anaerobically for Clostridium perfringens, with negative result.
Contributor's Diagnosis and Comments: Colitis, severe, focally extensive, acute, erosive and necrotizing. Castor oil-induced superficial colitis, with secondary probable endotoxin-induced submucosal thrombosis, inflammation, and mucosal necrosis.
A large area of the submucosa is thickened by edema, hemorrhage, acute inflammatory cell infiltration, lymphatic distention and venous and capillary thrombosis. The overlying mucosa in necrotic, and there are multiple surface clumps of bacteria. Adjacent viable areas of mucosa have flattened superficial epithelium, inflammatory cells between membrane and epithelium and capillary thrombosis. Severe hemorrhagic foci are present in the muscularis and serosa.
Acute superficial enterocolitis is reported in the literature following the experimental administration of castor oil to ponies. In the cecum and ventral colon, there is extensive erosion of superficial epithelium between crypts, with fibrin, neutrophils and cell debris on the denuded basement membrane. By 48 hours post ingestion, there is partial restitution of surface epithelium by flattened epithelial cells extending from the necks of crypts. Fragmented necrotic debris is sometimes present within macrophages within the lamina propria. Mucosal venules beneath the erosions are plugged with fibrin, and there is general mucosal venous congestion and distention of submucosal lymphatics.
In the equine gastrointestinal system, transit is rapid from stomach to cecum to ventral colon, but slow from ventral colon to dorsal colon, allowing prolonged exposure to toxic components. The toxic component of castor oil is ricinoleic acid. The full thickness necrosis of the mucosa and the severity of the submucosal lesion in this case suggest additional factors were operating as well as castor oil. After uncomplicated poisoning by castor oil, it is reported that repair of the mucosa is complete by 72 hours post-dosing.
Disruption of the epithelium caused by castor oil may have allowed absorption of bacterial endotoxin from the lumen of the intestine. Endotoxin has widespread effects, including those on macrophages, endothelium, platelets, Hageman factor, neutrophils, complement and mesenchymal cells. Macrophages become activated, to produce interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-à). These cytokines have effects on endothelium and leucocytes: endothelium synthesizes adhesion molecules, other cytokines, growth factors, eicosanoids, nitric oxide, and it increases in thrombogenicity. Reactive oxygen species produced in the respiratory burst of activated macrophages have toxic effects, such as cell membrane lysis and extracellular membrane degradation. Endotoxin damages endothelium directly or indirectly, exposing subendothelial collagen, and initiating the coagulation cascade by the intrinsic pathway. The extrinsic pathway is activated by thromboplastin-like procoagulant activity (PCA) expressed by endothelial cells and activated macrophages. Endotoxin stimulates platelets to make phospholipid more available for coagulation, and to synthesize and release thromboxane A2 , which induces platelet aggregation. Endotoxin activates factor XII (Hageman factor), and the alternate pathway of complement. Neutrophils are directly primed by endotoxin to increase their response to mediators; they are attracted by IL-1, complement and leukotrienes, activated by TNF- à and complement. Endotoxin stimulates mesenchymal cells to release proteolytic enzymes.
Other diseases of the equine colon featuring erosion of epithelium, mucosal and submucosal edema, inflammatory cell infiltration and thrombosis are acute salmonellosis, colitis X, Potomac horse fever, and Clostridium perfringens type A enterotoxemia.
AFIP Diagnosis: Colon: Colitis, necro-ulcerative, acute, focally extensive, severe, with hemorrhage, fibrin thrombi and fibrinoid vascular necrosis, Arabian, equine.
Conference Note: Diseases such as acute salmonellosis, colitis X, antibiotic- induced diarrhea, Potomac horse fever, and endotoxemia are characterized by a loss of mucosal integrity; inflammation of the mucosa and submucosa of the ileum, cecum, and large colon; depletion of peripheral leukocytes; and fluid, electrolyte and acid-base derangements (secretion acidosis, with low serum HCO3- , high serum Cl-, and a normal anion gap). These shared features suggest shared pathophysiologic mechanisms. Castor-oil induced colitis and diarrhea has been suggested as a model for acute equine colitis and could be utilized to study the pathophysiologic mechanisms involved in colitis-associated diarrhea.
Contributor: Laboratory of Large Animal Pathology, New Bolton Center, University of Pennsylvania, 382 West Street Road, Kennett Square, PA 19348-1692.
References:
1. Cotran RS, Kumar V, Robbins SL: Robbins Pathologic Basis of
Disease, 5th ed. Pp 70-71. WB Saunders, Philadelphia, 1994.
2. Kumar V, Cotran RS, Robbins SL: Basic pathology, 5th ed. Pp. 78-79. WB Saunders, Philadelphia, 1992.
3. Johnson CM, Cullen JM, Roberts MC: Morphologic characterization of castor oil-induced colitis in ponies. Vet Path 30:248-255, 1993.
4. MacKay RJ: Endotoxemia. In Current Therapy in Equine Medicine 3, Robinson NE, ed pp. 225-232. WB Saunders, Philadelphia, 1992.
5. Morris DD: Endotoxemia in horses. A review of cellular and humoral mediators involved in its pathogenesis. J Vet Internal Med 5:167-181, 1991.
6. Roberts MC, Clarke LL, Johnson CM: Castor oil-induced diarrhea in ponies: a Model for acute colitis. Equine Vet J 21(suppl. 7):60-67, 1989.
International Veterinary Pathology Slide Bank: Laser disc frame #21256.
Signalment: 19-year-old castrated male Arabian horse.
History: Diagnosed with equine protozoal myelitis 60 days before euthanasia based on clinical signs, clinical pathology and positive test for anti-Sarcocystis antibody in cerebral spinal fluid (Western blot). The horse was treated and improved moderately. After treatment was stopped, the horse deteriorated and the owners elected euthanasia.
Gross Pathology: Extending from cervical spinal cord segment 6 (C6) to thoracic spinal cord segment 3 (T3), there is an extensive area of dark discoloration (malacia). On the left side, malacic areas include the medial and ventral areas of the spinal white matter and extend into the left ventral horn of the grey matter. On the right side, the area of myelomalacia is smaller and restricted to the ventral funiculi, but also extends into the medial-ventral aspect of the ventral horn of the grey matter. There are no significant gross lesions evident in the brain, other areas of spinal cord or other organs.
Laboratory Results: None submitted.
Contributor's Diagnosis and Comments: In locally extensive areas of the spinal cord (S1), corresponding to the areas where the gross lesions were identified, there are large numbers of lymphocytes and macrophages, with local infiltrates of neutrophils and isolated eosinophils surrounding leptomeningeal vessels and extending into the subjacent neuropil of both the grey and white matter. Vessel walls, within both the white and grey matter, are infiltrated and surrounded by concentric accumulations of an eosinophilic, amorphous material which shows apple-green birefringence under polarized light after Congo-red staining (amyloid). Within some sections, there is an extensive accumulation of amyloid surrounding the central canal. There is intense gliosis of both white and grey matter. Within the white matter there is extensive, multifocal axonal degeneration and large numbers of axon sheaths containing gitter cells. Within the grey matter there is neuronal satellitosis and neuronal degeneration and necrosis characterized by swollen neurons which show extensive chromolysis. Multifocally, within the meninges there are moderate perivascular accumulations of lymphocytes and plasma cells with smaller numbers of neutrophils (not evident in all sections).
The most severe necrotizing and inflammatory lesions with perivascular amyloid are centered around T1 and T2; however, a gradation of inflammatory lesions extends from C5-T4. A second small, less severe, focus of myelitis, which unilaterally involves the left ventral grey horn and extends into the white matter, is also present at L1-L2.
The lesions in the spinal cord are consistent with protozoal myelitis (Sarcocystis neurona); however, no protozoal cysts or individual zoites could be definitively identified in the sections examined. This may be the result of the extensive long term therapy with a folic acid antagonist. The extensive accumulation of perivascular amyloid in the spinal cord is a very unusual lesion. Cerebral perivascular amyloid is described in old dogs and is associated with neuronal senile plaques. The cerebral amyloid deposits in old dogs are immunoreactive for beta-amyloid protein found typically in human brain from patients with Alzheimer s disease. Spinal cord amyloid angiopathy has been described in the human literature but is considered a rare and unusual lesion. Amyloid deposition, usually of the AA type (serum amyloid protein), is not uncommonly found in other parenchymal organs (liver and kidney) associated with chronic inflammatory diseases, but to our knowledge has not been described in the CNS in horses. The exclusive localization of the amyloid to the areas of spinal cord with the most severe inflammatory lesions and not to other areas of CNS or parenchymal origins suggests that its genesis is likely secondary to the local inflammatory response. However, a genesis independent of the inflammatory lesion cannot be definitively ruled out.
AFIP Diagnosis: Spinal cord: Myelitis, nonsuppurative, diffuse, moderate, with axonal degeneration and perivascular and multifocal amyloid, Arabian, equine.
Conference Note: Sarcocystis has an obligatory two-host life cycle. Carnivores (the definitive hosts) become infected by ingesting infective cysts in the muscle of birds or mammalian herbivores. Zoites from the cysts invade the intestinal epithelium and develop directly into gamonts. Oocysts sporulate within the intestine and are shed as infective sporocysts in the feces of the carnivore. Sporozoites are released after ingestion of sporocysts by susceptible herbivorous mammals or birds. They migrate to arterial vessels and develop into meronts within endothelial cells. Merozoites from these meronts initiate a second generation of meronts in capillaries throughout the body. Merozoites from these second generation meronts enter mononuclear cells in the circulation and divide by endodyogeny. These merozoites enter heart and skeletal muscle cells and neural tissue where they develop into noninfective sarcocysts that contain unicellular metrocytes. Metrocytes then produce bradyzoites that are infective for the predator animal. The horse is a dead-end host for S. neurona (S. falcatula). Meronts are found within the cytoplasm of neuronal cells, leukocytes, and multinucleate giant cells in the brain and spinal cord.
Small subunit ribosomal RNA sequencing of Sarcocystis falcatula, a protozoan that cycles between opossums (Didelphis virginiana) and birds, and S. neurona indicates that the organisms are one and the same. This implies that opossums and birds infected with S. falcatula serve as a reservoir for the protozoa causing protozal myelitis in horses.
Contributor: University of Illinois, College of Veterinary Medicine, 2001 South Lincoln Ave, Urbana, Illinois 61801.
References:
1. Okuda R; Uchida K; Tateyama S; Yamaguchi R; Nakayama H; Goto
N. The distribution of amyloid beta precursor protein in canine
brain. Acta Neuropathol (Berl) 1994; 87(2):161-7.
2. Tokuda T; Ikeda S; Maruyama K; Yanagisawa N: Ito N. Sinal cord vascular and leptomengeal amyloid beta-protein deposition in a case with cerebral amyloid angiopathy. Acta Neuropathol (Berl) 1992;84(2):207-10.
3. Uchida K; Nakayama H; Tateyama S; Goto N. Immunohistochemical analysis of constituents of senile plaques and cerebro-vascular amyloid in aged dogs. J Vet Med Sci 1992;54(5):1023-9.
4. Dame JB, MacKay RJ, et al: Sarcocystis falcatula from passerine and psittacine birds: synonymy with Sarcocystis neurona, agent of equine protozoal myeloencephalitis. J Parasitol, 81(6):930-5, 1995.
5. Fenger CK, Granstrom DE, et al: Identification of opossums (Didelphis virginiana) as the putative definitive host of Sarcocystis neurona. J Parasitol, 81 (6):916-9, 1995.
6. Gardiner CH, Fayer R, Dubey JP: An atlas of protozoan parasites in animal tissues. United States Department of Agriculture, Agricultural handbook #651, pp. 40- 45, 1988.
International Veterinary Pathology Slide Bank: Laser disc frame #554, 1056, 1566, 2126, 2227, 3868-70, 5685, 7496-99, 7775- 77, 11044-46, 12727, 19102, 19106, and 24038-40.
Signalment: 3-day-old female Standardbred equine.
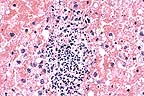 Suppurative adrenalitis in
a foal with Actinobacillus equuli septicemia. (HE, 400X, 70K).
Suppurative adrenalitis in
a foal with Actinobacillus equuli septicemia. (HE, 400X, 70K).
History: Foal born 4/16/94 to a maiden mare. Foal was term and the delivery was normal according to the owner. The foal nursed normally. The mare dripped milk only for a few hours prior to foaling. At presentation, the foal s temperature was 107oF; the foal was comatose and had nystagmus. Blood glucose was 14 mg/dl, and the foal was acidotic, tachypneic, and had very low blood pressure. The foal died 2 hours after arrival.
Antemortem diagnosis: Septicemia
Gross Pathology: Kidneys: Multifocal severe acute suppurative nephritis. Adrenals: Multifocal acute suppurative adrenalitis Lungs: Pneumonia, presumptive Joints: Mild, acute suppurative synovitis
Diagnosis: Actinobacillus equuli pyosepticemia.
Laboratory Results: RBC 12.0 (6.3-9.2) mill/æl Bands 0.3 (0.00) thou/æl Total CO2 19 (28-37) mEq/L Calcium 10.1 (10.2-12.9) mg/dL Phosphorus 5.0 (2.0-4.6) mg/dL Total protein 3.8 (6.1-8.0) g/dL Creatinine 3.1 (1.0-2.0) mg/dL AST 161 (193-509) U/L Alkaline phos 2395 (80-287) U/L Anion gap 22 (6-15) mEq/L pH 7.23 (7.32-7.44) Bicarb 19 (24-30) mM/L
Contributor's Diagnosis and Comments:
1. Liver: Multifocally, distributed in the hepatic parenchyma, there is a moderate number of variably sized microabscesses. 2. Kidneys: The cortical tissue contains numerous glomeruli undergoing necrosis caused by a severe multifocal suppurative infiltrate. Numerous glomeruli are completely obscured by suppurative infiltrates characterized by degenerate neutrophils and necrotic debris. 3. Adrenal gland: The adrenal cortex is diffusely, severely hemorrhagic and contains a moderate number of small foci of necrosuppurative inflammation.
AFIP Diagnosis: Adrenal gland, cortex: Necrosis and hemorrhage, diffuse, with multifocal suppurative adrenal adenitis and capsular fibrin thrombi, Standardbred, equine.
Conference Note: Actinobacillus equuli is a gram-negative, facultative anaerobic bacillus. It is found worldwide and is a normal inhabitant of the equine intestine. Actinobacillus equuli primarily affects immunosuppressed foals or those with failure of passive transfer. In the foal, A. equuli causes fibrinopurulent arthritis, omphalophlebitis, suppurative nephritis, pneumonia, and abscesses in many other organs. This organism is also an important cause of fetal death and abortion in horses.
The hemorrhage and thrombosis in this case were most likely caused by endotoxins. The effects of endotoxins on endothelial cells and the resultant damage and initiation of the coagulation cascade is discussed in detail in Case 1 of this conference.
Differential diagnosis for septicemia in the foal includes Escherichia coli, Staphylococcus, Streptococcus, Salmonella, and Actinobacillus. Definitive diagnosis cannot be made without culture.
Contributor: Cornell University - New York State, College of Veterinary Medicine - Dept of Pathology.
References:
1. Koterba AM, Brewer BD, and Tarplee FA: Clinical and clinicopathological
characteristics of the septicaemic neonatal foal: Review of 38
cases. Equine Vet. J., 16(4):376-383, 1984.
2. Platt H: Septicaemia in the foal. A review of 61 cases. Br Vet J 129(3):221- 29, 1973.
3. McGuire TC, Crawford TB, Hallowell AL, and Macomber LE: Failure of colostral immunoglobulin transfer as an explanation for most infections and deaths of neonatal foals. JAVMA, 170(1): 1302-4, 1977.
International Veterinary Pathology Slide Bank: Laser disc frame #428-9, 1593, 3049-51, 3864-67, 4125, 5237-40, 5274, 6248, 9473, 22313-15, and 22869-71.
Signalment: 11-year-old dapple gray German Warmblood.
History: The horse was presented to the clinic because of apathy of 2 months duration and conjunctivitis with periorbital edema of approximately 4 weeks duration. Five days prior to admittance, therapy resistant fever and leucocytosis had been diagnosed by the attending veterinarian.
At admission, the horse was in poor body condition and apathetic. Rectal temperature was 39.8 øC, heart rate was 44/min., and respiratory rate was 16/min.
The conjunctivitis and periorbital edema were evident, along with increased lacrimal discharge.
The nares, lips and vulva were also edematous; the nostrils and lips had numerous reddish-gray skin fissures of variable size which were covered with a dry yellowish serous secretion. The lip mucosa was edematous and had small (0.5 cm) to mid-sized (5 cm) lesions. The mandibular lymph nodes were enlarged (fist-sized): 7x8 cm) and dolent.
Gross Pathology: The horse had skin lesions of variable size (0.5-2.5 cm) distributed over the head, especially concentrated on the lips and nostrils. The lesions were reddish, crusty and greasy. The gingiva had multiple mucosal fissures, and round mucosal defects (0.5-1 cm).
All available lymph nodes were enlarged and the retropharyngeal lymph nodes were extremely large (7 cm in diameter), with a white-brown cut surface.
Heart: The atrioventricular and the semilunar valves were enlarged and thickened, with large nodules (2-3 cm in diameter, gelatinous to solid in consistency, white to brown color). The walls of the aorta and the pulmonary artery had irregular cut surfaces, with diffuse, small nodules scattered through the intima.
All intestinal lymph nodes were enlarged with white-brownish cut surfaces. The taeniae coli and colon mucosa were gelatinous and enlarged.
The cut surface of the kidneys revealed diffuse irregular white areas in the cortex and medulla.
Laboratory Results: Blood sedimentation: 88mm/15min.(-15mm/15min.) Fibrinogen: 6g/l (1-5g.l) Leucocytes: 28000/ l (5055-9470/ l) Lymphocytes: 21918/ l (962-3770/ l) Bilirubin: 95.9 mol/l (17-46,6 mol/l) Creatinine: 222 mol/l 81-127 mol/l)
Contributor's Diagnosis and Comments: Skin: Cutaneous epitheliotropic lymphoma (mycosis fungoides). Granulomatous to necrotizing superficial dermatitis with bacteria and fungi. Heart: Lymphoma.
The skin section was taken from the area of the muzzle. The superficial dermis is mildly infiltrated with atypical lymphocytes. They vary in size and they have hyper- convoluted vesicular to hyperchromatic nuclei. Some of these lymphoid cells appear blastic with large vesicular rounded nuclei and prominent nucleoli. Mitotic figures are abundant. The cellular infiltrate consists of some small lymphocytes, neutrophils, macrophages and multinucleated giant cells. The infiltrates of atypical lymphocytes extend into the deep dermis and the subcutis. Furthermore, the lymphoid infiltrate could be found in the epidermis and in the epithelium of hair follicles and adnexal structures. In some places, the intra-epithelial infiltrations form clusters (Pautrier s microabscesses). The dermis is thickened; acanthosis, hyper- and parakeratosis are present. There is focal epidermal necrosis and bacteria and fungal structures (yeasts) are also seen.
The heart tissue sample originates from the heart base. The epicardial fatty tissue and the outer layers of the myocardium are highly infiltrated by atypical lymphocytes, which are similar to those found in the skin.
Lymphoid infiltrations were also found in the valves, in the intima of the aorta and the pulmonary artery, in the lung, mesenteric and retropharyneal lymph nodes, spleen, liver, kidneys and the large intestine.
Most neoplastic cells were CD3-positive and could be identified as neoplastic T- lymphocytes. This is a consistent finding in humans (Theil and Leuschner, 1985) and dogs with mycosis fungoides (Ferrer et al., 1993). However, in humans the neoplastic cells are mostly CD4-positive (Ferrer et al., 1993) whereas in dogs they are reported to be predominantly positive for CD8 (Moore et al., 1994). The monoclonal antibodies CD4 and CD8 could not be used because no frozen tissue was available.
The etiology of mycosis fungoides in not known. In humans a continuous antigen influence and/or a defect of Langerhans cells are suppected to induce a long lasting activation and subsequently a neoplastic transformation of T-lymphocytes (Gross et al., 1992). In humans, cutaneous epithelitropic lymphomas are called mycosis fungoides because in the late stage, illness the skin tumors resemble mushrooms (Thiel and Leuscherm, 1985). Similar lymphoproliferative diseases are described in dogs (Thiel and Leuschner, 1985;Wilcock, 1989;Ferrer et al., 1993; Moore et al., 1994), cats (Baker and Scott, 1989) and cattle (Zwahlen et al., 1987). Cutaneous lymphomas are also reported in horses (Jubb et al., 1993).
In this case, the diagnosis of epitheliotrophic lymphosarcoma ( mycosis fungoides ) was based on the following findings: atypical lymphocytes with clefted nuclei, intra-epithelial lymphoid infiltrations arranged singly or in clusters (Pautrier s micro abscesses). Metastasis in various organs is consistent with the late stage of the illness.
AFIP Diagnosis: 1. Skin: Lymphosarcoma, epitheliotrophic, German Warmblood, equine. 2. Heart: Lymphosarcoma.
Conference Note: In the dog, epitheliotropic lymphosarcoma often presents as solitary or multiple mucocutaneous and perioral ulcerated plaques; generalized scaling erythroderma and cutaneous nodules are the other common clinical presentations. The lesions begin as erythematous, alopecic plaques that progress to firm, non-ulcerated nodules with or without pruritus. Ultrastructurally, the most characteristic feature of the neoplastic T-lymphocytes is an irregular nuclear membrane. In humans, a small number of cutaneous T-cell lymphomas have cells with hyperconvoluted, cerebriform nuclei; these are known as Sezary cells.
In instances where a single cutaneous mass is present, the prognosis for recovery is good if the tumor is completely excised. If multiple cutaneous lesions are present, the prognosis is poor. Metastasis to lymph nodes, liver, spleen, kidney, lung, and tonsil is common.
Contributor: Institute Fr Veterin rpathologic, Wintertherstr. 968, 8057 Z rich, Switzerland.
References:
1. W. Thiel und G. Leuschner (1985): Kasuistik: šber eine
Hauterkrankung beim Hund „hnlich der menschlichen Mycosis
fungoides, kleinteirpraxis 30, 253-258. Verlag M. & H. Schaper,
Hannover.
2. G. Jaeschke und R. Rudolph (1985): Die Leukose des Pferdes: 1. Nomenklatur, Klinik und Pathologie (šbersichtsreferat); Berl. M
nch. Tier„rztl. Wschr. 98,088-094 (1985).
3. Jerome S. Burke, MD, Khalil Sheibani, MD and Henry Rappaport, MD (1986): Dermatopathic Lymphadenopathy. An Immunophenotypic Comparison of Cases Associated and Unassociated With Mycosis Fungoids. AJP, Vol.123, No. 2 may 1986.
4. R. D. Zwahlen, A. Tontis, and A. Schneider (1987): Cutaneous Lymphosarcoma of helper/Inducer T-Cell Origin in a Calf, Vet. Pathol. 24:504-508 (1987).
5. Joyce L. Baker, DVM and Danny W. Scott, DVM (1989): Mycosis fungoides in two cats. Journal of the American Animal Hospital Association; January/February 1989, Vol. 25.
6. Brian P. Wilcock, Julie A. Yager (1989): The behavior of epidermotropic lymphoma in twenty-five dogs. Can. Vet. J., Vol 30, September 1989.
7. P. G. Detilleux, N. F. Cheville and B. J. Sheahan (1989): Ultrastructure and lectin histochemistry of equine cutaneous histiolymphocytic lymphosarcomas. Vet. Pathol. 26: 409-419 (1989).
8. Jack E. Moulton and John W. Harvey (1990): Tumors in Domestic Animals. Third Edition, University of California Press (1990).
9. Martin O. Furr, DVM; Mark V. Crisman, DVM,MS; John Robertson, DVM, PhD; Ota Barta, DVM, PhD; William S. Swecker, Jr., DVM, PhD. (1992): Immunodeficiency associated with lymphosarcoma in a horse J.A.V.M.A., Vol, 201, No 2, July 15, 1992.
10. Thelma Lee Gross, Peter J. Ihrke: Emily J. Walder (1992): Veterinary Dermatopathology. First Edition.
11. K.V.F. Jubb, Peter C. Kennedy, and Nigel Palmer (1993): Pathology of Domestic Animals. The hematopoietic system. Vol. 3: 151-152, Fourth Edition (1993).
12. Luis Ferrer, Dolores Fondevila, Rosa Rabanal, Jordi Tarres, Antonio Ramis (1993): Immunohistochemical detection of CD3 antigen (pan T marker) in canine lymphomas. J. Vet. Diagn. Invest. 5:616-620 (1993).
13. Peter F. Moore, Thierra Olivry and Diane Naydan (1994): Animal model: canine cutaneous epitheliotrophic lymphoma (mycosis fungoides) is a proliferative disorder of CD8+ T cells. Am J Pathol, Vol. 144, No. 2, February 1994.
International Veterinary Pathology Slide Bank: Laser disc frame #7921, 11015-17, 11555, 11782, 11786, 11802-3, 11807-8, 21381-2, and 21551.
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.