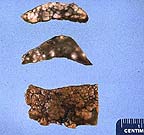
 Multilobular appearance in
the liver of a cat on both the capsular surface and on cut sections.
The nodules are firm, white, and elevated. (34K)
Multilobular appearance in
the liver of a cat on both the capsular surface and on cut sections.
The nodules are firm, white, and elevated. (34K)Signalment: 15-year-old spayed Domestic Shorthair cat.
History: Three and a half years prior to euthanasia this indoor cat had an episode of diarrhea which responded to Clindamycin therapy. The next few years involved intermittent bouts of inappetence and constipation followed by diarrhea, with gradual weight loss. The episodes responded to symptomatic therapy and Clindamycin. Immediately prior to euthanasia, she became acutely ill, stuporous, uncoordinated, and constipated but with a fairly good appetite. Unfortunately, she failed to respond to therapy this time and was euthanized.
Gross Pathology: The liver was enlarged and nodular with diffuse 3-5 mm irregular yellow-white slightly raised foci that extended from the surface into the parenchyma.
Laboratory Results: Fecal flotation performed during the initial episode of diarrhea revealed protozoal oocysts. The oocysts were sporulated by Dr. J.P. Dubey, USDA, and positively identified as Toxoplasma gondii. The cat's highest titer was >100,000. There was an inflammatory leukogram with dehydration, and mild elevation of ALT and AST. FeLV and FIV tests were negative. Additionally, hyperthyroidism and hyperadrenocorticism were ruled out.
During subsequent episodes, repeated fecal exams were negative, and laboratory results at various times showed elevated ALT, AST, total bilirubin, total protein and globulins with an inflammatory leukogram.
Contributor's Diagnosis and Comments: Liver: Cholangiohepatitis, lymphoplasmacytic, chronic, periportal, diffuse and bridging, with nodular hyperplasia and marked bile duct proliferation, Domestic Shorthair cat, feline.
Feline cholangitis/cholangiohepatitis syndrome occurs in three histologic forms: suppurative cholangitis/cholangiohepatitis, long-term nonsuppurative cholangitis/cholangiohepatitis, and biliary cirrhosis. The three forms are thought to be progressive stages of the same disease. Initial inflammation of the biliary system spreads to the adjacent hepatic parenchyma. The long-term result is portal fibrosis and biliary hyperplasia. The etiology of the disease is unknown, although immune-mediated mechanisms have been proposed. Additionally, bacterial and parasitic infections (including toxoplasmosis) have been associated with suppurative cholangitis. Many cats with the disease respond favorably to corticosteroid and/or antibiotic therapy. Biochemical findings vary. Aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase enzymes are often high in the suppurative form, but can become normal as the disease progresses. Hyperbilirubinemia is common. In the present case, a causal relationship was not established between the toxoplasmosis and liver lesions.
AFIP Diagnosis: Liver: Biliary hyperplasia and fibrosis, bridging, diffuse, marked, with hepatocellular loss, nodular regeneration, and lymphoplasmacytic and neutrophilic cholangiohepatitis, Domestic Shorthair, feline.
Conference Note: Although the three histologic forms of feline cholangiohepatitis syndrome (suppurative, non-suppurative, and biliary cirrhosis) may represent a progression of lesions of the same cause, there are some inconsistancies with this theory. For example, while it might be assumed that the nonsuppurative form is a more chronic stage of the suppurative form, the nonsuppurative lesion seems to occur in a generally younger group of cats than the suppurative form.
Suppurative cholangitis/cholangiohepatitis is characterized by portal and parenchymal infiltration of neutrophils, accompanied by mild fibrosis and bile duct hyperplasia. Suppurative cholangiohepatitis is most common in middle-aged to old cats. Enteric bacteria are frequently isolated from these lesions and ascending bacterial infection of the bile duct has been proposed as the etiology; however, cholelithiasis, trematodes, the nephrotic syndrome, and protozoa have also been associated with suppurative cholangiohepatitis.
Nonsuppurative cholangiohepatitis is characterized by portal infiltrates of plasma cells and lymphocytes, bile duct hyperplasia, and periportal fibrosis; small numbers of neutrophils may be present. Nonsuppurative cholangiohepatitis has also been associated with pancreatitis. Cats less than 4 years of age are primarily affected.
Biliary cirrhosis is presumed to result from progressive cholangiohepatitis; however, the etiology is unknown. Histologically, there is prominent bridging portal fibrosis, bile duct hyperplasia, nodular hyperplasia, and chronic inflammation of varying severity. Regardless of etiology, the prognosis for any form of cholangiohepatitis is guarded due to a generally poor response to therapy.
Some attending the conference diagnosed cholangiocarcinoma; however, there are several features that argue against a neoplastic process. At the center of most of the areas of bile duct hyperplasia, there are pre-existent portal structures, suggesting a diffuse proliferative lesion rather than widespread portal invasion of neoplastic cells. Additionally, there is little cellular atypia of the proliferating epithelial cells, the proliferating ducts are linedby a single layer of cells, and the mitotic rate is low. Also, nodular hepatocellular regeneration and biliary hyperplasia are not commonly associated with cholangiocarcinoma.
Contributor: Walter Reed Army Institute of Research, Washington, D.C. 20307-5100.
References:
1. Day DG: Feline cholangiohepatitis complex. Vet Clin North Am
Small Anim Pract 25(2):375-85, 1995.
2. Jackson MW. Panciera DL, Hartmann F: Administration of vancomycin for treatment of ascending bacterial cholangiohepatitis in a cat. JAVMA 204(4):602-5, 1994.
3. Kelly WR: The liver and biliary system, In: Pathology of Domestic Animals, Jubb KVF, Kennedy PC, Palmer N. Eds., 4th Ed, Volume 2, Academic Press, Orlando, FL, 360-362, 1993.
4. Morrison WB: Cholangitis, choledocholithiasis, and icterus in a cat. Vet Path 22:285-286, 1985.
5. Prasse KW et al: Chronic lymphocytic cholangitis in three cats. Vet Path 19:99-108, 1982.
International Veterinary Pathology Slide Bank: Laser disc frame #5433-7, 13096, 16357, and 16360-1.
Signalment: 14-month-old male Tennessee Walking Horse.
History: This horse was presented to the Tuskegee University School of Veterinary Medicine's large animal hospital. The animal had a 3 month history of hind limb ataxia. Before being admitted to the hospital, the horse went down and did not get up. Radiographs revealed compression of the cervical spinal cord at C3. The owner elected euthanasia and the horse was presented for necropsy.
Gross Pathology: There was compression of the cervical spinal cord along with narrowing of the vertebral canal at the juncture of C3 and C4. There was a ventral protuberance that originated from the dorsal surface of the bone of C3 together with an increased amount of fibrocartilaginous material at the articulation between C3 and C4. The ventrolateral wall of the vertebral canal at the point of compression was reddened.
Laboratory Results: none submitted.
Contributor's Diagnosis and Comments: Vacuolar leukomyelopathy, with axonal degeneration, subacute, locally extensive, bilaterally symmetrical, moderate to severe, ventral and lateral white columns, cervical spinal cord, equine.
This section of spinal cord is characterized by diffuse, bilaterally symmetrical, vacuolation of the ventral and lateral white columns. Many of the vacuoles contain moderately to markedly swollen axons. Fragmented axons are also noted in some of the vacuoles. Vasculature is prominent throughout the affected area.
Cervical vertebral stenotic myelopathy in horses causes incoordination, hind limb ataxia, and locomotor disturbances. These clinical signs are most frequently due to stenosis of the vertebral canal at C3-C4. Vertebral changes may be responsible for clinical signs by damaging the spinal cord when the neck is ventroflexed. Lesions are often present in articular processes as a result of the degenerative arthropathy that is secondary to osteochondrosis.
The disease is usually present in horses 12-15 months of age, but may be diagnosed as late as 4 years of age. Young, fast growing male thoroughbreds are most commonly affected.
AFIP Diagnosis: Spinal cord, cervical, white matter: Degeneration and loss, axonal, diffuse, severe, with dilated myelin sheaths, axonal swelling, and axonophagia, Tennessee Walking Horse, equine.
Conference Note: Cervical vertebral stenotic myelopathy of horses is a condition caused by compression of the cervical spinal cord. It is not progressive; however, recovery from severe deficits does not occur. The condition includes two syndromes, cervical vertebral instability and cervical static stenosis.
Cervical vertebral instability causes narrowing of the spinal canal when the neck is flexed. Lesions commonly occurs at C3-5 in fast-growing, male thoroughbreds. Affected vertebrae are malformed; the floor of the spinal canal angles upward while the roof angles downward, narrowing the spinal canal. The etiology of cervical vertebral instability has not been determined; however, there is evidence that implicates rapid growth, feeding of high-protein and high-energy rations and the development of cervical vertebral instability. These same factors are attributed to the development of osteochondrosis and many affected horses also have this disease.
Cervical static stenosis is also common in large male horses; however, it primarily affects horses 1 to 4 years of age, and position of the neck does not influence the degree of spinal compression. Cervical static stenosis is caused by thickening of the ligamentum flavum and the dorsal laminae of the vertebral arches. The thickening of the ligaments is caused by formation of fibrocartilage in the ligaments and joint capsules. Thickening of the dorsal laminae is due to osteosclerosis. The osteosclerosis and fibrocartilaginous metaplasia of ligaments and joint capsules are consistent with increased mechanical force and range of motion.
There are often no gross lesions in the spinal cord. Occasionally, there is poliomalacia at the point of compression. There may also be a slight depression in the contour of the spinal cord at the site of compression. Histologically, the white matter is more severely affected than the grey matter. At the site of compression there is loss of myelin and axonal degeneration. In severe cases, there may be hemorrhage and necrosis of the grey matter in the ventral or lateral horns. Cranial to the point of compression, there is axonal degeneration and loss of myelin of the ascending fibers in the dorsal funiculi. Conversely, the descending tracts in the ventral and ventrolateral funiculi degenerate caudal to the point of compression.
Other diseases that must be differentiated from cervical vertebral stenotic myelopathy include protozoal myeloencephalitis, equine herpesvirus-1 infection, equine degenerative myeloencephalitis, vertebral abscessation, synovial cysts, and traumatic lesions.
A similar condition, cervical vertebral malformation-malarticulation, is seen in young, rapidly growing dogs. This disorder occurs most frequently in the Doberman Pinscher and Great Dane, although other large breed dogs are sometimes affected. The compressive lesion frequently occurs at C6-C7; otherwise, the gross and histologic manifestations of the syndrome are comparable to those described for the horse.
Contributor: Tuskegee University School of Veterinary Medicine, Dept. of Pathology and Parasitology, Tuskegee University, Tuskegee, AL 36088.
References:
1. Jubb, KVF, Kennedy, PC, and Palmer, N. Bones and Joints. In:
Pathology of Domestic Animals, 3rd ed. Academic Press, Inc., San
Diego, new York, Berkeley, Boston, London, Sydney, Tokyo, Toronto.
Pp. 31-35, 1985.
2. Tomizawa, N et. al. Relationship Between Radiography of Cervical Cord to Wobbling 19 Foals. J. Vet. Med. Sci. (Japan). 56(2):227-233, 1994.
Signalment: One-year-old female Holsteiner equine.
History: Presented with colic. Exploratory surgery revealed a thickened distal 6 feet of small intestine. The affected bowel was resected and submitted for histopathology. The animal subsequently was euthanized.
Gross Pathology: The affected portion of small intestine is thickened and has prominent folding of the mucosa.
Laboratory Results: None submitted.
Contributor's Diagnosis and Comments: Small intestine; enteritis, proliferative, diffuse, severe, equine, with intracellular Campylobacter-like organisms.
This is a case of proliferative enteritis which occurs rarely in the horse and has been reported only once. The disease occurs in several animal species, most commonly in swine. The disease in all species appears to be caused by the same or similar organism and originally was considered to be a Campylobacter-like species. Recently, the organism in swine has been shown to be a new species unrelated to Campylobacter and named Ileobacter. It is likely that this organism will be found to be the cause of proliferative enteritis in all species.
AFIP Diagnosis: Small intestine: Enteritis, proliferative, subacute, diffuse, moderate, with villous atrophy and submucosal edema, Holsteiner, equine.
Conference Note: Proliferative enteritis has been described in swine (intestinal adenomatosis), hamsters (wet-tail), puppies (proliferative ileitis), a foal (intestinal adenomatosis), guinea pigs (duodenal hyperplasia), rabbits (enterotyphlocolitis), blue foxes (intestinal adenomatosis), and ferrets (proliferative colitis). It has not been determined if proliferative enteritis is caused by the same agent in each of these species or if different etiologies exist; however, the disease can be transmitted to hamsters from swine.
The causative agent of proliferative enteritis in swine was identified as Lawsonia intracellularis in 1995. This agent has previously been known as Campylobacter sputorum, Campylobacter-like organism, and most recently as Ileal Symbiont intracellularis. Lawsonia intracellularis replicates within the apical cytoplasm of epithelial cells of the ileum in pigs. The bacteria are not membrane bound and occur free in the cytoplasm. Organisms do not stain with with Gram's stains or acid-fast stains and are PAS-negative but can be demonstrated with silver stains. Immunohistologic staining suggests that L. intracellularis is related to the organisms that cause proliferative enteritis in other species.
Histologically, the lesions consist of mucosal epithelial hyperplasia and varying amounts of crypt inflammation. In the present case, there are crypt herniation and crypt abscess formation in some sections. There is a paucity of goblet cells and blunting of the villi. The Warthin-Starry procedure demonstrated large numbers of argyrophilic bacilli in the apical cytoplasm of enterocytes. Bacilli are also visible free in the cytoplasm of enterocytes in the electron micrograph submitted by contributor.
Contributor: Department of Biomedical Sciences and Pathobiology, College of Veterinary Medicine, Virginia Tech, Blacksburg, VA 246061-0442.
References:
1. Duhamel GE, Wheeldon EB. Intestinal Adenomatosis in a Foal.
Veterinary Pathology 19:447-450, 1982.
2. Barker IK, van Dreumel AA, Palmer N. The Alimentary System. In: Pathology of Domestic Animals, 4th ed. Jubb, Kennedy, Palmer (eds). Academic Press, Inc. San Diego, p233, 1993.
3. Moore, GM and Shryock TR: Lawsonia intracellularis and swine enteric disease. Comp Con Ed, 18(1): s11-s17, 1996.
International Veterinary Pathology Slide Bank: Laser disc frame #1841, 1903-5, 2816, 9259, and 12929.
Signalment: 8-year-old Hanoverian gelding horse.
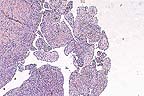 Papillary proliferations of
neoplastic mesothelium in a Hanoverian gelding. (HE, 40X, 64K)
Papillary proliferations of
neoplastic mesothelium in a Hanoverian gelding. (HE, 40X, 64K)
History: The horse was submitted to an animal hospital with severe ascites and mild colic. Ultrasonographical investigation revealed an irregular liver surface. The animal was euthanized.
Gross Pathology: At necropsy, the subcutaneous tissue and all lymph nodes were severely edematous. The peritoneal cavity was filled with approximately 80 liters of a yellowish, clear fluid. The serosal surfaces of all abdominal organs, including the parietal peritoneum, were covered with numerous, gray-white, firm nodules ranging from 1 mm to 5 cm in diameter. The surface of the nodules was either smooth or cauliflower-like. There were no macroscopically visible masses in the abdominal lymph nodes. There were no gross or microscopic lesions in the thoracic cavity including lung, heart or pericardium.
Laboratory Results: None submitted.
Contributor's Diagnosis and Comments: Malignant biphasic peritoneal mesothelioma.
Histologically, the periphery of nodules consisted of epithelioid neoplastic mesothelial cells with squamous, cuboidal or columnar appearance forming papillary projections. In these cells, mitotic figures were frequent. Cells often sloughed from the surface of the nodules into the abdominal lumen. Occasionally, binucleated cells were observed. Inflammatory cells were diffusely scattered throughout the neoplastic tissue. The neoplastic nodules were well vascularized. The centers of the projections consisted of neoplastic mesothelial cells which were fibroblastic to mesenchymal in appearance. In some areas, there was an abundant accumulation of extracellular matrix.
Ultrastructurally, the cells in the periphery of the nodules had numerous microvilli and desmosomes. Within the nodules, neoplastic mesothelial cells were located in a collagen-rich matrix. The tissue had a sarcomatous appearance. These cells formed desmosomes as did the epithelioid neoplastic mesothelial cells in the periphery.
Peritoneal mesotheliomas are rarely seen in horses. In animals they are most frequent in cattle. Mesotheliomas arise from cells of mesodermal origin and grow in an epithelioid and mesenchymal pattern, as observed in the present case. The neoplasm must be classified as malignant because of its widespread occurrence on all abdominal serosal surfaces and the number of mitotic figures in the nodules. However, the tumor did not invade the underlying tissue and there was no metastasis to regional lymph nodes. Human mesotheliomas are classified as epithelial, sarcomatous and biphasic (mixed) depending on the predominant histological feature. In the present case, both epithelial and mesenchymal characteristics were observed. The tumor was, therefore, classified as malignant biphasic peritoneal mesothelioma.
AFIP Diagnosis: Serosal surface and attached skeletal muscle: Mesothelioma, Hanoverian, equine.
Conference Note: Mesotheliomas occur with the greatest frequency in cattle and dogs, but have been reported in most domestic species. In cattle, they occur most often as a congenital neoplasm in fetuses or young calves. Mesotheliomas arise from the serous lining of pericardial, pleural, and peritoneal cavities. They routinely spread by direct extension, regularly invade underlying tissue, but rarely metastasize to other organs; when dissemination does occur, it appears to be hematogenous. Mesothelioma arising from the tunica vaginalis is one of the most common tumors of the male Fischer 344 rat.
Grossly, mesotheliomas appear as multiple firm, sessile, or pedunculated nodules arising from a serosal surface. The nodules range in size from a few millimeters to 10 centimeters in diameter. The tumors may cause the accumulation of large amounts of fluid, resulting in ascites, cardiac insufficency, cardiac tamponade, or respiratory distress depending upon the location of the tumor. Adhesions are often formed between the affected serosal surface and adjacent organs.
Histologically, mesotheliomas exhibit a broad spectrum of patterns from mostly epithelioid to predominantly fibrous. The most common pattern consists of neoplastic cuboidal epithelioid cells covering papillary projections of neoplastic spindle cells which form a fibrovascular core. The fibrovascular core may appear sarcomatous and contains an extracellular matrix that is weakly metachromatic with Alcian blue, methyl violet, or toluidine blue stains. The metachromatic staining is lost after digestion with testicular hyaluronidase; therefore, the material is most likely hyaluronic acid. Ultrastructurally, neoplastic mesothelial cells have a prominent basal lamina, well developed microvilli, desmosomes, abundant rough endoplasmic reticulum, and mitochondria. Immunohistochemical staining is positive for keratin and sporadically positive for vimentin.
Contributor: Institute f
r Patholgie, Tier„rztliche Hochschule Hannover, B
nteweg 17, 30559 Hannover, Germany.
References:
1. Carnine, B.L., Schneider, G., Cook, J.E., Leipold, H.W. (1977):
Pericardial mesothelioma in a horse. Vet. Pathol. 14, 513-515.
2. Colbourne, C.M., Bolton, J.R., Mills, J., Whitaker, D., Yovich, J.V., Howell, J. McM. (1992): Mesothelioma in horses. Aust. Vet. J. 69:275-278.
3. Rickets, S.W. Peace, C.K. (1976): A case of peritoneal mesothelioma in a thoroughbred mare. Equine Vet. J. 8, 78-80.
4. Wallace, S.S., Jayo. M.J., Maddux, J.M., DeBowers, R.M., Leipold, H.W. (1987): Mesothelioma in a horse. Comp. Cont. Educ. 9, 21-216.
5. Barker IK: The peritoneum and retroperitoneum in Pathology of Domestic Animals. Jubb, KVF, Kennedy, PC, and Palmer (eds.) 4th ed. Academic Press, Inc., San Diego. Vol. 2, pp. 31-35, 1993.
International Veterinary Pathology Slide Bank: Laser disc frame #155-7, 648-9, 742, 1053, 2067, 2867-8, 2896, 3598, 5665, 6873, 8829-33, 9072, 10901, 10903, 12072, 12721, 13953, 13958-9, 15869-72, 20082-3, 20303, 20883, 20938, and 23552.
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.