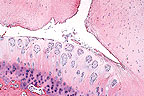
 Cleft formation, fibrillation,
and formation of chondrones in the articular cartilage of a thoroughbred
horse with osteochondrosis of the cervical vertebra (HE, 100X,
87K)
Cleft formation, fibrillation,
and formation of chondrones in the articular cartilage of a thoroughbred
horse with osteochondrosis of the cervical vertebra (HE, 100X,
87K)Case I - 89-42 (AFIP 2506721)
Signalment: Fifteen-month-old male thoroughbred (88-2757). Nine-month-old male thoroughbred (89-42).
History: These horses were part of a study that examined the frequency and severity of lesions considered to be osteochondrosis in horses with cervical stenotic myelopathy. 88-2757 had no clinical signs related to the nervous or musculoskeletal system and was utilized as a control. 89-42 had severe ataxia and evidence of dynamic compression of the spinal canal at C3-C4 on myelogram.
Gross Pathology: 88-2757 is from the margin of posterior left articular facet of C2. This was a focal area of smooth and intact but slightly irregular articular cartilage. 89-42 is from the margin of anterior left C3, this was a focal area of thin and slightly roughened articular cartilage.
Laboratory Results: None submitted.
Contributor's Diagnosis and Comments:
88-2757: Morphologic diagnosis - Necrosis and cleft formation at articular - epiphyseal complex with resorption and fibrous replacement of subchondral bone. Name the disease - osteochondrosis. Etiology - unknown. 89-42: Morphologic diagnosis - Necrosis and cleft formation within epiphyseal and articular cartilage with chondrone formation and fibrillation. Name the disease - osteochondrosis with secondary degenerative joint disease. Etiology - unknown.
Slides from both cases were needed to achieve the number of slides required and it is not intended that the lesions be compared and correlated with clinical signs. Most slides are from the control horse to emphasize the nature of lesions that can occur symptomatically. In this particular study, severity but not frequency of lesions of osteochondrosis in cervical vertebrae was greater in horses with cervical stenotic myelopathy than in the controls. The frequency and severity of osteochondrosis in the appendicular skeleton were both greater in horses with cervical sites of compression but compression also occurred at sites with no lesions. Most lesions of osteochondrosis in cervical facets have areas of retention of growth cartilage with variable invaginations. The lesion present on these two slides represents necrosis and cleft formation without apparent cartilage retention. This is considered a form of osteochondrosis and has been associated with copper deficiency, zinc excess and cadmium excess in horses. The role of these deficiencies in the current cases in unknown. The resorption of subchondral bone and fibrosis in 88-2757 and the chondrone formation and fibrillation in 89-42 are considered secondary.
AFIP Diagnosis: Cervical vertebra, articular facet: Necrosis and cleft formation, focally extensive, moderate, with chondrone formation and subchondral fibrosis, Thoroughbred, equine.
Conference Note: Osteochondrosis affects young animals and is characterized by abnormalities in the growth cartilage of the articular-epiphyseal cartilage complex and the physeal growth plate. Species commonly affected by osteochondrosis include the pig, dog, horse, chicken, turkey, humans, and to a lesser extent, sheep and cattle. Several factors, such as inheritance, growth rates, physical activity, gender, and nutrition, have been associated with the development of osteochondrosis. Osteochondrosis is not a primary disease of articular cartilage. Secondary involvement of articular cartilage is a common sequelae of osteochondrosis and results in arthropathy, osteochondritis dissecans, or areas of collapse of the articular cartilage. Microscopic lesions commonly associated with osteochondrosis include the formation of eosinophilic streaks, metaphyseal dysplasia, and cartilage necrosis.
Eosinophilic streaks are normally present in articular cartilage and increase with age. They are considered to be vestiges of cartilage canals. Normal streaks are arranged in parallel to cartilage columns; in osteochondrosis they are often stellate and are associated with areas of cartilage necrosis or areas of metaphyseal dysplasia.
Metaphyseal dysplasia is the focal persistence of proliferative and/or hypertrophic chondrocytes. Persistence of these cells results in the formation of a cone-shaped thickening of the growth plate. The chondrocytes in these areas fail to maintain the normal columnar architecture of the normal growth plate. Areas of chondrodysplasia either undergo normal ossification, persist as nodules of cartilage in the metaphysis, or are replaced by areas of fibrocartilage or woven bone.
Cartilage necrosis is most common in the articular-epiphyseal complex. Necrosis of cartilage is thought to be an early change and may not be present by the time clinical signs are evident. Necrotic cartilage may be by-passed during endochondral ossification and remain in the epiphysis or metaphysis; however, necrosis often leads to the development of osteochondritis dissecans. Osteochondritis dissecans is characterized by the formation of a cleft of articular cartilage that frequently appears to be dissected away from underlying bone. The cartilage flap usually remains partially attached; if it is completely separated it becomes a "joint mouse". The clefts form over or near areas of necrosis in the articular-epiphyseal complex. The cleft formation does not involve bone; however, the flaps of cartilage may develop areas of osseous metaplasia.
In the horse, osteochondrosis occurs more commonly in the sagittal ridge of the distal tibia, the articular processes of cervical vertebrae, the medial condyle and the trochlear ridges of the femur, the patella, and the tarsal and fetlock joints. The cause of osteochondrosis in horses is unknown. Experimental and field cases of osteochondrosis have been associated with copper deficiency and numerous compounds that influence copper availability, including excess zinc, excess calcium, and dexamethasone. Copper is a catalyst for lysyl oxidase, a protein required for formation of collagen cross-links. Cartilage from copper deficient animals has been shown to have a high proportion of soluble collagen and is more susceptible to trauma. Copper supplementation in foals has reduced, but not prevented, the incidence of osteochondrosis.
Contributor: The Ohio State University, Dept. Of Veterinary Biosciences, 1925 Coffey Rd, Columbus, OH 43210-1093.
References:
1. Bridges, C.H., Womack, J.E., Harris, E.D., and Scrutchfield,
W.L. Consideration of copper metabolism in osteochondrosis of
suckling foals JAVMA 185:173-178, 1984.
2. Bridges, C.H., and Harris E.D. Experimentally induced cartilaginous
fractures (osteochondritis dissecans) in foals fed low-copper
diets. JAVMA 193:215-221, 1988.
3. Gunson, D.E., Kowalczyk, D.F., Shoop, C.R., and Ramberg, C.F.
Environmental zinc and cadmium pollution associated with generalized
osteochondrosis, osteoporosis, and nephrocalcinosis in horses.
JAVMA 180:295-299, 1982.
4. Jeffcott, L.B. Osteochondrosis in the horse - searching for
this key to pathogenesis. Equine Vet. J. 23:331-338, 1991.
5. Knight, D.A., Weisbrode, S.E., Schmall, L.M., Reed, S.M., Gabel,
A.A., Bramlage, L.R., and Tyznik, W.I. The effects of copper supplementation
on the prevalence of cartilage lesion in foals. Equine Vet. J.
22:426-432, 1990.
6. Stewart, R.H., Reed, S.M., and Weisbrode, S.E. Frequency and
severity of osteochondrosis in horses with cervical stenotic myelopathy.
Am. J. Vet. Res. 52:873:879, 1991.
7. Palmer, N: Bones and joints in Pathology of Domestic Animals.
Jubb KVF, Kennedy PC, and Palmer N eds., 4th edition, Academic
Press, Inc. San Diego, pp. 118-125, 1993.
International Veterinary Pathology Slide Bank: Laser disc frame
#2484, 15273, 15322, and 24651-2.
Case II - 34525 (AFIP 2503650), 2 photos
Signalment: Spayed female Irish Terrier mix canine. The dog was 17«-months- old and weighed 45 lbs at first presentation.
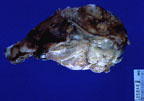 Large greyish white neoplasm
infiltrating the femur of an Irish terrier (50K).
Large greyish white neoplasm
infiltrating the femur of an Irish terrier (50K).
History: The animal was presented on 5/10/95 after a three week history of left hind leg lameness. There had been no improvement with aspirin therapy. The distal left femur was slightly swollen and painful on palpation. Radiographs revealed extensive lysis and proliferation involving the distal left femur. A bone biopsy was done. Cultures were taken at the veterinary clinic and tissues were submitted for histopathological evaluation to the Division of Comparative Medicine at John Hopkins Medical Institutions. On the basis of the histopathological diagnosis, amputation of the limb was recommended. A midshaft amputation of left femur was done 5/31/95 and the leg was submitted to John Hopkins. Chest films and limited routine blood chemistries done at this time revealed no abnormalities. The owner reported that the dog was active and alert for several weeks post surgery; however, on 6/19/95, the animal was readmitted. It had been anorectic for 4-5 days, had lost weight (overall weight now 32 lbs), had pale, icteric mucous membranes and a fluctuant abdomen. There was also a soft, lobulated, fleshy mass, 1 cm in diameter, protruding from the gum line of the lower right canine tooth. Chest radiographs were unremarkable. An enlarged liver was noted on abdominal radiographs; however, the remainder of the abdomen was obscured by fluid densities. The owner elected euthanasia for the pet and a necropsy was conducted.
Gross Pathology: Leg: A large, proliferative, soft, grey-white, glistening (fatty appearance) multilobulated mass encompassed the entire distal left femur. A marked periosteal reaction was present extending from the mass along the shaft of the bone and ending several centimeters below the amputation site. On cut surface there was considerable replacement of bone by the previously described tissue mass and protrusion of the mass beyond borders of the bone. There were multiple foci of hemorrhage and necrosis as well. Small multilobulated masses were noted near the margins of the patella and multifocally distributed among the cruciate ligaments. Skeletal muscle surrounding the limb was extremely atrophied.
Laboratory Results:
Evaluation of biopsy specimens revealed abundant necrotic material, a few small irregular bone spicules and several small to moderately-sized rafts of large vacuolated (signet ring like) mononuclear cells with round or ovoid nuclei. Cytoplasmic vacuoles were variably sized and well delineated. Occasional mitotic figures were noted. Oil-red-O stains on sections from the femoral mass revealed lipid droplets within the cytoplasm of neoplastic cells. Preliminary limited blood work done at the veterinary clinic revealed no abnormalities. Cultures taken from the bone biopsy and done at the clinic were negative. Fluid analysis of the pleural and peritoneal fluids revealed high white counts with a predominance of lymphocytes. Abnormal cells were noted in pleural cytology smears.
Contributor's Diagnosis and Comments: Tumor, bone, distal femur, liposarcoma of bone (marrow), Irish Terrier mix, canine.
Histological evaluation of sections from the femoral mass revealed extensive replacement of marrow, trabecular bone and destruction of cortical bone by an expansile mass composed predominately of large round to oval cells with vacuolated cytoplasm. Vacuoles were well delineated and variable in size and number. Occasional mitotic figures were noted. Scattered throughout were smaller foci of fibrous, myxomatous and osteosarcomatous differentiation and areas of hemorrhage and necrosis. Hepatic, renal and pulmonary masses consisted of the same type of abnormal vacuolated cell population described previously. Greater amounts of necrotic tissue were present in hepatic and renal masses.
Liposarcoma of bone (marrow) is an extremely rare tumor. In the veterinary literature there have been only a handful of published reports and one anecdotal case of spontaneously occurring tumors in dogs and one of a spontaneous tumor in a kudu. Of the cases report in dogs, all animals have been young (16-18 months) and only long bones were involved with metastases to lung, liver and spleen. There was no breed predilection. In experimental studies using 239Pu in young beagles, numerous skeletal malignancies were induced; one was diagnosed as a liposarcoma of bone.
Liposarcoma of bone is also a rare tumor in human medicine. It is noted to be a neoplasia of adults with most cases occuring between the ages of 25-45 years. There is no sex predilection, and the most frequent sites are the tibia, femur and humerus. A certain amount of debate centers on establishing criteria for determining the origin of the malignant cells. Arguments for origin in bone are based on observation that liposarcoma arising in the soft tissue adjacent to bone rarely infiltrate the bone and that when invasion does occur, deep infiltration is unusual. In this case, there was extensive involvement and deep penetration into the bone suggesting that the tumor was of bone marrow origin.
AFIP Diagnosis: Femur: Liposarcoma, Irish Terrier cross, canine.
Conference Note: Liposarcoma of bone is a primary bone tumor that arises from adipocyte precursors in the marrow cavity. Liposarcoma of bone closely resembles its soft-tissue counterpart and is composed of signet ring cells and multivesicular cells. The neoplasm may contain areas of fibrosarcomatous differentiation or undifferentiated spindle-cell sarcoma. Fat stains should demonstrate the presence of fat in the neoplastic cells (confirmed in this case). The differential diagnosis includes central fibrosarcoma of bone, central lipoma of bone, and liposarcoma of soft-tissues with metastasis to bone.
Contributor: Division of Comparative Medicine, Department of Pathology, Johns Hopkins University, Baltimore, MD 21205.
References:
1. Brodey, RS, and Riser, WH: Liposarcoma of Bone: Case report.
J. Amer. Vet. Radiol. Soc 7:27-33, 1966.
2. Davis, PE, Dixon, RT, Johnson, JA, and Paris, R: Multiple liposarcoma
of bone marrow origin in a Greyhound. J. Small Anim. Pract. 15:445-456,
1974.
3. Downey, EF Jr, Worsham, GF, and Brower, AC: Liposarcoma of
bone with osteosarcomatous foci: Case report and review of the
literature. Skeletal Radiol. 8:47-50, 1982.
4. Pardo-Mindan, FJ, Ayala, H, Joly, M, Gimeno, E, and Vazquez,
JJ: Primary liposarcoma of bone: Light and electron microscopic
study. Cancer 48(2):274-280, 1984.
5. Lloyd, RD, Taylor, GN, Angus, W, Bruenger, FW, and Miller,
SC: Bone cancer occurrence among beagles given 239Pu as young
adults. Health Physics 64(1):45-51.
6. Moulton, JE: Tumors in Domestic Animals. 3rd ed, University
of California Press, Berkeley, CA. Pp. 213-214, 1990.
7. Raubenheimer, EJ, van Heerden, J, Keffen, RH, and Lemmer, LB:
Liposarcoma of bone marrow origin in a Kudu. J. Wildlife Dis.
26(2):271-274, 1990.
International Veterinary Pathology Slide Bank: Laser disc frame #22697, 24063, and 24064-6.
Case III - S-39 (AFIP 2508343)
Signalment: 28-day-old male Sprague-Dawley rat (Tac:N(SD)fBR).
History: This 28-day-old Sprague-Dawley rat was a subject in a bisphosphonate efficacy study. A bisphosphonate compound was administered subcutaneously to the rat for 7 days prior to euthanasia. The animals were fed ad libitum a standard certified laboratory rodent diet containing not Less than 23.0% crude protein. This rat was euthanatized at the termination of the study. Specimens were fixed in 70% ethanol, embedded in methyl methacrylate, and sectioned undecalcified. Slides were stained with toluidine blue.
Gross Pathology: None submitted.
Laboratory Results: None submitted.
Contributor's Diagnosis and Comments: Tibia: Hyperostosis, metaphyseal, mild, subacute.
Mineralized bone is stained pale blue. Growth plate cartilage and retained cartilage cores (metaphysis) are stained deep purple. The cortical bone may appear thicker than normal and the medullary cavity narrowed in some slides due to section of cut.
There has been an arrest of spongiosa resorption beneath the growth plate in this rat. Osteoclasts are hyper-nucleated and appear more numerous than in the controls. The dose administered to this rat creates lesions similar in appearance to osteopetrosis, which is characterized by deficient resorption of spongiosa and remodeling/modeling deficits in the metaphysis. Note that in contrast to resorption of spongiosa, the resorption of cartilage below the hypertrophic zone of the growth plate is normal (as in hereditary osteopetrosis). This observation supports the concept that cartilage resorption may be mediated by vascular in-growth rather than by osteoclasts/chondroclasts.
Bisphosphonates are analogs of pyrophosphate and as a class, inhibit osteoclastic resorption and remodeling of bone. The phosphorous-oxygen bond of pyrophosphate (P--O--P) has been replaced with a phosphorus carbon bond (P--C--P), which is poorly metabolized. The side chain attached to the central carbon alters properties such as anti-mineralization and anti-resorption activity. Bisphosphonates have a high affinity for mineralized tissue and are incorporated on any exposed bone surface. This specific targeting to osteoclast resorption pits and/or mineralizing bone surfaces provides high local concentrations at the desired site of action. The mechanism of action of bisphosphonates is currently unknown. Hypotheses vary from direct inhibition at the cell surface to inhibition of intracellular metabolism by incorporation in methylene-containing adenine nucleotides. Recent studies of the structure-activity relationship for the bisphosphonate side chain indicate, however, that at least the newer generations of nitrogen-containing bisphosphonates probably act by binding to a specific target at a site that is complementary in structure to the bisphosphonate side chain.
Bisphosphonates are used in the treatment of accelerated bone turnover diseases. Bisphosphonates are currently approved in the United States for treatment of Paget's Disease and heterotopic ossification. They are approved for treatment of osteoporosis in Europe. The treatment population does not include patients with open growth plates.
AFIP Diagnosis: Tibia: Hyperostosis, metaphyseal, diffuse, mild to moderate, with persistent cartilage cores and increased numbers of osteoclasts, Sprague-Dawley rat, rodent.
Conference Note: There are many terms that are utilized to indicate an increase in skeletal bone mass including osteosclerosis, panosteitis, osteopetrosis, osseous hyperplasia, etc. Many of these terms are used to signify specific diseases in certain species such as canine panosteitis. Utilization of these terms in morphologic diagnoses can be confusing. To prevent misinterpretation of these terms, criteria have been established for rats by the Society of Toxicologic Pathologists and are published in Proliferative Lesions of Bone, Cartilage, Tooth, and Synovium in Rats in Guides for Toxicologic Pathology. This Guide for the rat defines hyperostosis as an abnormal increase of non-neoplastic skeletal bone mass; it may be proliferative or non-proliferative. Additional bone mass caused by increased bone formation and /or increases in numbers of bone cells is considered proliferative; an example would be callus formation at the site of a fracture. Increased bone mass due to a decrease in bone resorption is non-proliferative, as occurred in this case of bisphosphonate induced hyperostosis.
Maintenance of the shape of a bone is integrated with growth of the animal by a process known as modeling. Modeling requires synchronized activity of osteoclasts and osteoblasts, with either resorption or formation of bone at a given site, but never both simultaneously. The net result is movement of bone through space, as can be demonstrated in the modeling of primary spongiosa into secondary spongiosa. The modeling skeleton contains a high proportion of young bone which is less mineralized and can produce new trabeculae by endochondral ossification. The process of modeling can be compared with remodeling, which is the process of skeletal renewal throughout life. In this process, bone is replaced in the same location by the same type of bone.
In the case of bisphosphonate administration, there is a failure of modeling. The mineralized cartilage of the primary spongiosa is not properly resorbed, resulting in retained cartilage cores within the trabeculae and persistence of medullary bone. The pattern formed by these affected trabeculae has been described as a growth-retardation lattice. Similar histologic patterns in skeletal bone can be seen in cases of lead or phosphorous intoxication, or in cases of viral infections that primarily affect osteoclasts.
Contributor: Procter & Gamble Pharmaceuticals, Miami Valley Laboratories, P.O. Box 398707, Cincinnati, Ohio 45239-8707.
References:
1. Francis MD, Russell RGG, and Fleish H. Diphosphonates inhibit
hydroxyapatite dissolution in vitro and bone resorption in tissue
culture and in vivo. Science 65:1262-1264. (1969).
2. Schenk R, Merz WA, Mulbauer R, Russell RGG, and Fleisch H.
Effect of ethane-1 hydroxy-1,1-diphosphonate (EHDP) and dichloromethylene
diphosphonate (C12MDP) on the calcification and resorption of
cartilage and bone in the epiphysis and metaphysis of rats. Calcified
Tissue Int., 11:196, (1973).
3. Sietsema WK, Ebetino FH, Salvagno AM and Bevan JA. Antiresorptive
dose-response relationships across three generation of bisphosphonates.
Drugs Exptl. Clin. Res.XV(9):389-396, (1989).
4. Siesema WK, and Ebetino FH. Bisphosphonates in development
for metabolic bone disease: Exp. Opin. Invest. Drugs, 3(12):1255-1276,
(1994).
5. Rogers MJ, Xiong X, Brown RJ. Watts DJ, Russell RGG, Bayless
AV, Ebetino FH. Structure-activity relationships of new heterocycle-containing
bisphosphonates as inhibitors of bone resorption and as inhibitors
of growth of Dicyosteium discoideum amoebae. Mol. Pharmacol. 1995
47/2(398-402).
6. Palmer N: Bones and Joints in Pathology of Domestic Animals.
4th ed. Jubb KVF, Kennedy PC, and Palmer N, eds. Academic Press,
Inc., San Diego, pp. 17-22, 1993.
7. Long PH, Leininger JR, Nold JB, Lieuallen WG: Proliferative
lesions of bone, cartilage, tooth, and synovium in Rats in Guides
for Toxicologic Pathology. STP/ARP/AFIP, Washington DC, 1993.
Case IV - 95-00382 (AFIP 2507545)
Signalment: 12-year-old spayed female Bobtail dog.
History: Progressive lameness; sudden paraplegia with medullary compression in the Thoracic 4th-Thoracic 11th vertebral region associated with vertebrae, ribs, humeral and femoral osteolytic radiolucent foci; suspicion of multicentric bone tumors, possibly of multiple myeloma.
Gross Pathology: Pale mucous membranes (anemia). Intra-rachidian hematoma from the 7th to the 11th thoracic vertebras, compressive at the T-11 level; the 11th vertebra was partly destroyed by an osteolytic and hemorrhagic process. Hemorrhagic, 3 cm diameter, subpleural tumorous nodule beside the proximal part of the sixth left rib. Several other grey-white homogenous, moderately fibrous tumorous nodules were noted, including intraosseous masses in several ribs, the humerus, and femur. Some of the intraosseous nodules were difficult to differentiate from normal medullary tissue. There was one subcapsular hepatic nodule, 2 cm diameter, in the caudal part of the left lobe. In addition, there was congestive and hemorrhagic cystitis.
Laboratory Results: None submitted.
Contributor's Diagnosis and Comments: The presented tissue section is from a rib. The osseous-cartilaginous tissue of the rib is invaded by an osteolytic mass composed of two main types of cells:
The neoplastic mass is well vascularized by numerous congested, in some places cavernous, capillaries. Some limited areas of the mass show collagen formation.
Diagnosis: Giant-cell sarcoma (osteoclastoma), rib metastasis.
In spite of its reputation of being a very rare neoplasm in dogs and cats, giant-cell tumor (osteoclastoma) of bone is regularly reported in the literature (1,2,3,5,6,7,10,11,12,13). Locally aggressive, this type of tumor does not usually metastasize (9). In dogs, differential diagnosis is limited to giant cell reparative granuloma of bone (12). This type of tumor has been reported in horses (4,8). In this case, the primary site of the tumor was vertebral (11th thoracic vertebra) and was characterized by numerous metastatic nodules, mainly in ribs, humeral and femoral osseous tissue (the subcapsular liver nodule seen at necropsy, was histologically fibrous but, devoid of typical osteoclastic cells, was classified as a scar). The etiology is unknown in dogs. A retro-viral etiology has been reported for cats (10,13). The pathogenic role of various cytokines (transforming growth factor-á1, for example) in recruitment and migration of osteoclasts has recently be proposed for human giant cell tumors of bones (14).
AFIP Diagnosis: Rib: Giant cell tumor of bone, Bobtail (Old English Sheepdog), canine.
Conference Note: This case was reviewed by the Department of Orthopedic Pathology, AFIP. The mass is composed of two uniformly interspersed cell populations that invade surrounding bone. The predominant population is of polygonal to spindled stromal cells; the second population is of multinucleated giant cells. The neoplastic stromal cells form irregular, variably sized streams; some areas are solidly cellular. They have distinct cell borders and a moderate amount of eosinophilic fibrillar cytoplasm and are surrounded by a collagenous matrix. The nuclei of the stromal cells are round to oval with moderately stippled chromatin and an indistinct nucleolus; mitotic figures are infrequent. The multinucleate cells are large, up to 120 æm in diameter, with abundant eosinophilic granular cytoplasm. Nuclei are round with stippled chromatin and number from 3 to more than 50; mitotic figures are rare. The mass is partially bounded by pre-existing bone. There are multifocal areas of reactive osteoid production associated with the reactive bone; however, within the mass there is little osteoid present. Neoplastic stromal cells are immunohistochemically positive for vimentin. Both multinucleate giant cells and stromal cells are immunohistochemically negative for lysozyme.
Multinucleate giant cells may be present in many bone lesions including fibrosarcoma, osteosarcoma, reparative granuloma of bone, and in many infectious and toxic processes; therefore, the presence of multinucleate giant cells does not establish the diagnosis of giant cell tumor of bone. Metastasis of this tumor is unusual for giant cell tumor of bone and caused many to favor the diagnosis of osteosarcoma. In this case, the presence of a periosteal shell of reactive bone, the absence of cellular and nuclear atypia, a low mitotic rate, and the presence of little osteoid argue against osteosarcoma and are characteristic of giant cell tumor of bone. The giant cells of reparative granuloma of bone accumulate at the periphery of hemorrhages or around bone spicules; in giant cell tumor bone, on the other hand, multinucleate giant cells are scattered throughout the mass, as in this case. The multinucleated cells of human giant cell tumor of bone have immunohistochemical characteristics of osteoclasts. The spindled cells are postulated to be of bone marrow mesenchymal (stromal) cell origin, a population believed to be involved in the differentiation of osteoclasts from mononuclear precursor cells.
Contributor: Ecole V t rinaire d'Alfort, Laboratoire d'Anatomie Pathologique, 7, Avenue du G n ral de Gaulle, 94704, MAISONS ALFORT - FRANCE.
References:
1. Crow SE, Hall AD, Walshaw R & Wortman JA: Giant Cell
tumor (Osteoclastoma) in a Dog. J.A.A.H.A., (15):4, pp. 473-476,
1979.
2. De Martin BW, Grecchi R & Iwasaki M: Tumor De celulas na
Regiao Maxilo-Nasal E mandibular De Caes Dalmatas rev. Fac. Med.
Vet Zootec, Univ. S. Paulo, 10 pp. 45-52, 1973.
3. Garman RH: Malignant Giant Cell Tumor in a Dog. J.A.V.M.A.,
(171):6, 546-548,1973.
4. Gordon GE: Osteoclastoma in a Mare. Modern Vet. Practice, (55):7,
pp.540-549, 1974.
5. Gunsser I. Bildecricht. Osteoklastich-osteoplastiches Sarcom,
Berliner und Munchener Tierzatl,Woch., 1978,91,1,15.
6. Ianelli A., Cucinotta G.& Cartella I. Ulteriore contributo
alla conoscenza dei tumori ossei nel cane; su di uncaso di osteoclastoma
dell'estremita distale del radio. Annali della Facolta di Medicina
Veterinaria - Messina, 1985,22,41-54.
7. Lecouteur, RA, Nimmo J.S. Price SM & Pennock PW: A case
of Giant Cell Tumor of Bone (Osteclastoma) in a Dog J.A.A.H.A.,
(14):4, pp. 356-362, 1978.
8. Zheng MH, Fan Y, Wysockie SJ et al: Gene Expression of Transforming
Growth Factor-beta1 and its Type II Receptor in Giant Cell Tumors
of Bone. Am J of Pathol. (145): 5, pp. 1095-1104, 1994.
Dana P. Scott
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615
DSN: 662-2615
Internet: Scott@email.afip.osd.mil
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.