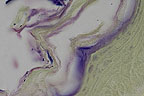
 Gram-positive corynebacteriform
bacteria enmeshed in hyperkeratotic debris from a hairless mouse
(Brown-Hopp's, 400X, 34K)
Gram-positive corynebacteriform
bacteria enmeshed in hyperkeratotic debris from a hairless mouse
(Brown-Hopp's, 400X, 34K)Signalment: 7-week-old female HSD: Athymic Nude - Nu mouse.
History: The mouse was one of 100 mice which appeared to be normal on arrival at 4 weeks of age. They were housed in flexible film isolators and subcutaneously implanted with an experimental human adenocarcinoma at 5 weeks of age. At 6-7 weeks of age, approximately « of the mice developed white flaky scaly changes on the skin of the face, back, sides and abdomen. This mouse was selected for necropsy and diagnostic work up.
Gross Pathology: The skin was crusty and thickened with white granular flaky areas on the back, abdomen, axilla and inguinal regions. The axillary lymph nodes were enlarged.
Laboratory Results: (Clinical pathology, microbiology, etc.)
The white cell count was 9700 with 49% neutrophils, 46% lymphocytes, 4% monocytes, and 1% eosinophils. A coryneform group D-2 bacterium and a few coagulase negative Staphylococcus species were isolated from culture of dorsal skin, ventral skin and enlarged axillary lymph node. No bacteria were isolated from culture of blood.
Contributor's Diagnosis and Comments:
Hyperkeratosis and acanthosis of Nude mice associated with coryneform bacteria.
This transient skin disease of nude mice has been observed for 10-15 years. Recently the disease was reproduced by Clifford et al. using a Corynebacterium species bacteria that was isolated from the skin of infected mice.
There is some controversy in the current methods of classifying Corynebacterium; perhaps more than one species of Corynebacterium are capable of producing the lesions. In this mouse, we also identified mild purulent lymphadenitis of the axillary lymph node; Corynebacterium group D-2 bacteria similar to those isolated from the skin were identified in the lymph node. None of the nude mice in this group with clinical signs became sick or died, although mortalities in suckling mice have been attributed to this organism by others. The clinical signs regressed spontaneously within a few weeks in all of the mice of this group, and the syndrome did not appear to affect the outcome of the study.
AFIP Diagnosis: Haired skin: Epidermal hyperplasia and orthokeratotic hyperkeratosis, diffuse, moderate, with moderate chronic-active dermatitis and steatitis, HSD:athymic Nude-Nu mouse, rodent.
Conference Note: Hyperkeratosis caused by hyperkeratosis-associated coryneform bacteria (HAC) has been described only in hairless mice, both athymic and euthymic; it has not been reported in hirsute strains. This suggests that hairlessness, rather than immunodeficiency, may be an important contributing factor in the development of the disease. The pathogenesis of the hyperkeratosis and acanthosis has not been determined.
Transmission of HAC occurs readily by direct contact, cohabitation, and by exposure to contaminated bedding. In addition, HAC infection is persistent; experimentally infected animals have remained culture positive for more than 33 days. Characteristic histologic lesions in association with gram-positive diphtheroids in the stratum corneum are highly suggestive of the disease; however, culture of the organism is necessary for definitive diagnosis.
Biochemical testing of HAC suggests that the organism is probably a new species of Corynebacterium, although the possibility that it may be a variant of C. bovis cannot be excluded. Definitive identification of HAC will be determined via RNA sequencing and DNA hybridization studies. Other Corynebacterium sp. commonly associated with disease in animals include C. kutscheri, the cause of pseudotuberculosis in rats; C. renale, which causes pyelonephritis in cattle; and C. ovis, a cause of abscesses and lymphadenitis in sheep and goats.
Contributor: The Milton S. Hershey Medical Center, Pennsylvania State Univ. Dept. Of Comparative Medicine, P.O. Box 850, Hershey, PA 17033.
References:
1. Clifford CB, Walton, BJ, Reed TH, et al.: Hyperkeratosis in
Athymic Nude Mice caused by a Coryneform Bacterium: Microbiology,
Transmission, Clinical Signs and Pathology. Lab Animal Science
45:131-139, 1995.
2. Percy DH, Barthold SW: Pathology of Laboratory Rodents and
Rabbits. Iowa State Press, Ames, pp. 38, 1993.
Signalment: 2-year-old female Holstein cow.
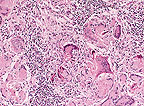 Granulomatous
dermatitis; multinucleate giant cells often contain mite fragments.
(HE, 400X, 156K)
Granulomatous
dermatitis; multinucleate giant cells often contain mite fragments.
(HE, 400X, 156K)
History: The owner noticed numerous elevated cutaneous masses, approximately 1.0 to 3.0 cm in diameter, distributed randomly on the body. An excisional biopsy of one mass was performed by the referring veterinarian.
Gross Pathology: The submitted sample of skin contained an elevated firm dermal mass containing a central accumulation of brown, soft material.
Contributor's Diagnosis and Comments:
1. Severe focal eosinophilic granulomatous parasitic dermatitis - Demodex spp. (Severe focal cutaneous demodecosis). 2. Moderate chronic eosinophilic perivascular dermatitis.
One section of skin was examined. Mild superficial hyperkeratosis was noted. Mild focal epidermal hyperplasia was also evident. Moderate perivascular accumulations of eosinophils intermixed with lymphocytes and occasional plasma cells were noted within the dermis. Mild to moderate apocrine gland dilation and dermal edema were also noted. One large focal elevated dermal mass was evident, composed of a central cystic site containing amorphous eosinophilic material intermixed with cellular debris and rimmed by large accumulations of lymphocytes intermixed with macrophages, plasma cells, eosinophils, epithelioid cells, multinucleated giant cells and immature fibrous connective tissue. Numerous elongated organisms were evident within the cystic center of the mass and within the adjacent inflammatory cellular infiltrate. The organisms were approximately 100 to 300 æm in length and contained numerous small basophilic spherical bodies internally. Many of the organisms had 4 pair of legs that appeared in the section as short eosinophilic appendages on the ventrolateral surface of the anterior aspect of the body. The mass appeared completely excised.
Demodectic mites are normal inhabitants of hair follicles or sebaceous glands of domestic animals and humans. Demodectic mange in cattle occurs worldwide and is produced by 3 Demodex species: D. bovis, D. ghanensis and one unnamed parasite. Gross lesions are characterized by multiple cutaneous nodules containing thick waxy material. The masses may rupture, producing an abscess or a granuloma within the skin. Histologically, the nodules represent follicular cysts that contain numerous demodex mites and keratin. Perforation or rupture of the follicular cyst can produce a severe focal granulomatous inflammatory reaction containing degenerating and mineralized segments of parasites rimmed by numerous lymphocytes, plasma cells, eosinophils, epithelioid cells and multinucleated giant cells.
AFIP Diagnosis: Haired skin: Granuloma, with eosinophils and central demodectic acarids, Holstein, bovine.
Conference Note: In cattle, demodecosis presents grossly as multiple cutaneous nodules over the shoulders, neck, dewlap, and muzzle. The number of nodules may vary from a few to several hundred. Histologically, the nodules are follicular cysts, lined by squamous epithelium, and filled with keratinous material and large numbers of mites. Rupture of the cyst induces a granulomatous reaction as seen in this case. Demodectic mange in cattle renders the hide unsuitable for leather production and is mainly of economic importance; however, in Africa and Madagascar, demodectic mange may be generalized and fatal.
Most animals are parasitized by a Demodex species. In dogs, Demodex canis most often causes a localized, self-limiting, lymphoplasmacytic perifolliculitis in dogs 3-10 months of age. Dogs that are immunosuppressed, either genetically or secondary to serious systemic disease, may develop a generalized form of demodectic mange that is complicated by bacteria, usually Staphylococcus. The generalized form of canine demodecosis can be fatal.
Cats rarely develop demodectic mange and when they do it is usually localized; however, a generalized form has been associated with feline leukemia virus infection, feline immunodeficiency virus infection, hyperadrenocorticism, and diabetes mellitus.
Demodecosis in large animals most often presents as nodular lesions similar to those described for cattle. Demodex caprae causes nodular lesions in goats, as does D. phylloides in pigs. Horses have two species of Demodex; D. caballi, which parasitizes the eyelids and muzzle; and D. equi, which can produce lesions anywhere on the body. Both species of demodex in horses induce a papular to nodular dermatitis similar to that described for cattle. Demodecosis is rare in sheep and is caused by either D. ovis or. D. aries.
Contributor: Animal Diagnostic Laboratory, Department of Veterinary Science, The Pennsylvania State University, University Park, PA 16802.
References:
1. Pathology of Domestic Animals, Jubb, KV.F., P.C. Kennedy, N.
Palmer (Eds.) 4th edition, Academic Press, Inc., Vol 1, pp. 686-690,
1993.
2. Fisher, W.F. Natural transmission of Demodex bovis stiles
in cattle. The Journal of Parasitology, Vol 59, No. 1, pp. 223-224,
February, 1973.
3. Murray, M.D., Demodectic mange of cattle, Australian Veterinary
Journal, Vol. 52. Pp. 49, January, 1976.
4. Matthes, H.F. Investigations of pathogenesis of cattle demodicosis:
sites of predilection, habitat and dynamics of demodectic nodules.
Veterinary Parasitology, 53, pp 283-291, 1994.
5. Parasitology for Veterinarians. Georgi, J.R. 3rd edition, W.B.
Saunders Company, pp. 64-65, 1980.
6. Parasitology, Maquardt, W.C., R.S. Demaree, Jr. Macmillan Publishing
Company, pp. 595-596. 1985.
International Veterinary Pathology Slide Bank: Laser disc frame #7228, 11813, 19522-3, 20583-4.
Signalment: Approximately 10-week-old female albino hairless lbm: MORO-hr (SPF) mouse.
 Multiple areas of erythema
and scaling on the back of a hairless lbm:MORO-hr mouse. (74K)
Multiple areas of erythema
and scaling on the back of a hairless lbm:MORO-hr mouse. (74K)
History: The animal received 8-methoxypsoralen (10mg/kg) orally by gavage and was exposed to artificial sunlight one hour after the administration (duration 15 minutes). Erythema was observed in the skin of the back as of day 2 after the exposure. The mouse was euthanized after 3 days.
Gross Pathology: Reddening and scale formation were observed in the skin of the back (area approximately 15 mm in diameter).
Laboratory Results: None submitted.
Contributor's Diagnosis and Comments:
1. Dermatitis, subacute, diffuse, moderate, focally pustulous, with epidermal cell degeneration and hyperplasia with cellular atypia (solar dermatosis), phototoxicity. 2. Folliculitis/furunculosis, subacute to chronic, moderate, with dilated hair follicles, hairless mouse.
The skin sections show the typical lesions induced by ultraviolet radiation. Epidermal changes include intracellular edema, particularly prominent in the stratum basale, hyperkeratosis (occasionally parakeratotic), hyperplasia with prominent atypia of epidermal cells and occasionally hyaline degeneration of keratinocytes with pyknotic nuclei. In some areas, clefts are formed at the dermo-epidermal junction. Occasionally, intraepidermal pustules are present, and in localized areas the entire epidermis is necrotic. In the dermis moderate mixed inflammatory cell infiltration is present which also extends to the adjacent subcutis. The skin of untreated mice exposed to the same light intensity showed no abnormalities. Therefore it can be concluded that the observed skin lesions represent a phototoxic reaction.
8-methoxypsoralen (xanthotoxin) is used for the treatment of human vitiligo and psoriasis and is a well known phototoxic agent. In toxicology it is frequently used as a positive control substance in phototoxicity studies to test the sensitivity of the animal strain. Phototoxicity is light-induced damage to the skin that does not rely on allergic mechanisms. The mechanism involves absorption of light by the compound itself, a metabolite or a complex of the compound with endogenous structures and reaction with DNA, RNA, proteins, or membranes. With 8-methoxypsoralen, skin toxicity correlates with the ability of the psoralen molecule to form DNA crosslinks. The severity of the lesion is proportional to the dose of the compound and the intensity of the radiation. In milder cases, mostly epidermal changes are observed such as intracellular edema and necrosis of keratinocytes. Characteristic is the presence of 'sunburn cells' (keratinocytes with hyaline cytoplasm and pyknotic nuclei), which occur in mice approximately 5 hrs after radiation. Later in the course hyperplastic changes develop: epidermal hyperplasia, parakeratosis, acanthosis. The hyperplastic changes reach a peak between 8 and 14 days. In pigmented skin tanning can be observed. Chronic skin reactions after prolonged exposure are parakeratotic hyperkeratosis, pseudoepitheliomatous hyperplasia, individual dyskeratotic cells and cellular atypia (solar keratosis) as well as solar elastosis. Long-term exposure may result in the development of skin neoplasms.
There are additional histological lesions in the skin sections apart from those described above related to phototoxicity. Hair follicles are dilated and occasionally filled with laminated keratin and there is a moderate granulomatous inflammation in the dermis associated with ruptured hair follicles. These changes are characteristic for hairless mouse strains, although in skin with phototoxic lesions they are usually more numerous than in untreated mice. Hairless mice are homozygous for the autosomal recessive gene 'hairless' and loose hair after growth of the first pelage. After a few weeks of age the animals are essentially naked except for scattered hairs from tylotrich follicles.
AFIP Diagnosis: 1. Haired skin: Squamous cell carcinoma in situ, mouse (albino hairless Ibm: MORO-hr strain), rodent. 2. Haired skin: Folliculitis and furunculosis, pyogranulomatous, diffuse, mild to moderate, with intraepithelial pustules, erosions, and ulcers.
Conference Note: The diagnosis of squamous cell carcinoma in situ is based on the significant degree of atypia of the epidermal and follicular epithelial cells, the presence of increased numbers of mitotic figures some of which are bizarre, anisokaryosis, anisocytosis, and multiple, irregularly shaped nucleoli. Neoplastic epidermal/follicular epithelial cells do not breech the basement membrane zone.
Most of the photobiologic reactions in the skin are induced by shortwave ultraviolet radiation in the 290 to 320 nm band (UV-B). This potentially harmful radiation is also referred to as actinic radiation. The acute effect of actinic radiation is sunburn, the mechanism of which is not well understood. It is believed that the immediate erythema develops due to direct heating of the skin and direct effects of UV-B radiation on dermal capillaries. The delayed and more severe erythematous reaction may be caused by direct damage to endothelial cells or may involve the release or production of cytokines from the UV radiation-damaged keratinocytes. The carcinogenicity of UV-B light stems from its ability to form pyrimidine dimers in DNA; when unrepaired these dimers lead to transcription errors and in some instances cancer. UV-B radiation may also cause mutations of oncogenes and tumor suppressor genes. In addition, UV-B radiation also induces production of suppressor T cells which further support tumor growth.
Photosensitization occurs when there is enhanced susceptibility of the skin to actinic radiation due to the presence of photodynamic agents. These agents absorb specific wavelengths of light, possibly beyond the UV-B range, thus making normally harmless radiation damaging. Activation of photodynamic agents induces a metastable triplet state; subsequent reaction with oxygen produces superoxide anion, singlet oxygen, and hydroxyl radicals. Oxygen free radicals are also produced by activation of xanthine oxidase in the skin. The reactive oxygen species damage nucleic acid, proteins, and lipoproteins.
Photodynamic agents generally reach the skin via hematogenous routes, although there are rare instances of percutaneous absorption. There are three categories of photosensitization classified according to the source of the agent. Type I photosensitization develops from exogenous photodynamic agents. Type II photosensitization is caused by aberrant endogenous pigment production, as is seen in bovine congenital erythropoietic porphyria. Type III photosensitization is also known as hepatogenous photosensitization. It is the most common form of photosensitization in animals and occurs when hepatic damage inhibits the liver's ability to secrete the photodynamic agent, for example phylloerythrin in ruminants. Phylloerythrin is formed from chlorophyll in the intestine and is normally excreted in bile.
Contributor: Ciba-Geigy AG, Preclinical Safety, Pathology, K-135.2.26, CH-4002, Basel, Switzerland.
References:
1. Benjamin S.A., Powers B.E., Nikula K.J., Hahn F.F.: Radiation
and Heat, Part II Ultraviolet Radiation. In: Haschek W.M., Rousseux
C.G. (eds.) Handbook of Toxicologic Pathology, Academic Press
Inc. San Diego (1991), pp. 1030-1039.
2. Dunnik J.K., Forbes P.D., Davies R.E., Iverson W.O.: Toxicity
of 8-methoxypsoralen, 5-Methoxypsoralen, 3-Carbethoxypsoralen,
5-Methylisopsoralen with Ultraviolet Radiation in the Hairless
(HRA/Skh) Mouse. Toxicology and Applied Pharmacology 89, 73-80
(1987).
3. Sundberg J.P.,Dunstan R.W., Compton J.G.: Hairless Mouse, HRS/j
hr/hr. In: Jones T.C., Mohr U., Hunt R.D. (Eds) ILSI Monographs
on Pathology of Laboratory Animals, Integument and Mammary Glands,
Springer-Verlag Berlin (1989), pp. 192-197.
4. Yager JA, and Scott DW: The Skin and Appendages in Pathology
of Domestic Animals. Jubb KVF, Kennedy PC, and Palmer N, eds.,
4th edition, Academic Press, Inc., San Diego, pp. 592-596, 1993.
International Veterinary Pathology Slide Bank: Laser disc frame #4030-1, 4396, 4399, 4979, 5347, 5658, 7345, 7416-8, 9468, 13161-4, 13702, 19568, 19570, and 22598-9.
Signalment: 10-week-old crossbred feeder pig.
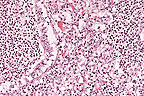 Suppurative bronchopneumonia
in a pig due to Actinobacillus suis. (HE, 200X, 125K)
Suppurative bronchopneumonia
in a pig due to Actinobacillus suis. (HE, 200X, 125K)
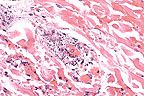 Necrotizing vasculitis in
the subcutis of a pig with Actinobacillus suis septicemia.
(HE, 200X, 85K)
Necrotizing vasculitis in
the subcutis of a pig with Actinobacillus suis septicemia.
(HE, 200X, 85K)
History: These pigs originated from a high health status herd. The farm was experiencing increased incidence of death loss in pigs 10 to 14 weeks old.
Gross Pathology: Necropsy examination of the affected pigs showed acute fibrinosuppurative bronchopneumonia and pleuritis. Most of the affected pigs had dark reddish-blue discoloration of the ear margins along with multiple 1 to 3 cm well demarcated dark reddish-blue cutaneous lesions distributed mainly along the ventral and lateral thorax and abdomen. Thoracic and abdominal cavities often contained turbid yellow fluid and strands of fibrin. Joints often contained similar appearing fluid. The spleens were enlarged and meaty.
Laboratory Results: Actinobacillus suis was isolated from the lungs.
Contributor's Diagnosis and Comments:
Skin - Multifocal acute suppurative necrotizing vasculitis. Lung - Diffuse acute suppurative bronchopneumonia.
Actinobacillus suis presents with differing clinical syndromes in a swine herd. Disease can cause acute death in neonatal piglets with typical signs of septicemia. Older swine may show septicemic disease or have pneumonia which is grossly and microscopically indistinguishable from Actinobacillus pleuropneumonia. Some animals may show more of an embolic pneumonia. Actinobacillus suis has also been implicated in causing abortions. Cutaneous lesions from Actinobacillus suis can look very similar to those seen with erysipelas. The lesions have a similar etiology in that the acute necrotizing vasculitis is often associated with septic thrombi.
In this particular herd, Actinobacillus associated disease has been seen at all levels of production including adult breeding animals, 5- to 10-day-old suckling pigs, nursery pigs and finishing swine up to 200 pounds. Actinobacillus suis produces APX 1 and APX 2 toxins which are similar to those produced by Actinobacillus pleuropneumonia. Actinobacillus suis can cross react with some serologic tests for Actinobacillus pleuropneumonia. Recent work suggests that neutralizing antibodies directed toward the Actinobacillus suis exotoxins may provide protection against disease caused by Actinobacillus pleuropneumonia.
AFIP Diagnosis: 1. Haired skin and subcutis: Vasculitis, necrotizing, suppurative, multifocal, moderate, with focally extensive epidermal necrosis, cross-breed, porcine. 2. Lung: Bronchopneumonia, necrotizing, suppurative, diffuse, severe, with colonies of coccobacilli.
Conference Note: Actinobacillus suis is a commensal organism found in the tonsil and vagina of pigs. Infection with A. suis probably spreads to the respiratory tract from the tonsil. Production of large numbers of bacteria and the release of exotoxins results in a fibrinonecrotic pleuropneumonia and vasculitis that cannot be distinguished histologically from the lesions of porcine pleuropneumonia, caused by A. pleuropneumonia. Bacterial thromboemboli are frequently widespread and may occur in any organ. Bacterial emboli in cutaneous vessels produce vasculitis and infarction of the skin that is similar to that seen in erysipelas. In contrast, extrapulmonary vascular lesions are not a predominant component of porcine pleuropneumonia and, when they do occur, develop primarily in the kidney .
Actinobacillus pleuropneumoniae (once classified as Haemophilus pleruopneumonia) produces exotoxins that belong to the RTX family. Three toxins, ApxI, ApxII, and ApxIII have been demonstrated. In addition, exotoxins similar to ApxI and ApxII have been found in strains of A. suis. ApxI toxin is strongly hemolytic and cytotoxic. ApxII toxin is weakly hemolytic and moderately cytotoxic. ApxIII toxin is nonhemolytic but strongly cytotoxic. Field strains of A. pleuropneumonia and A. suis have been shown to secrete either ApxI, ApxII, ApxI and ApxII, or ApxII and ApxIII. The variation of types of exotoxins secreted and the amounts produced may mediate the varied pathogenicity of different strains of Actinobacillus.
Contributor: College of Veterinary Medicine, Mississippi State University, Box 9825, Mississippi State, MS 39762.
References: 1. Fenwick, B. Protection against porcine pleuropneumonia (App) provided by previous infection with Actinobacillus suis. Proceedings. American Association of Swine Practitioners. 365-366. 1995.
2. Miniats, O.P., M. R. Spinato, S.E. Sanford. Actinobacillus suis septicemia in mature swine: Two outbreaks resembling erysipelas. Can. Vet J. 30:943-947. 1989.
3. Kamp, E.M., T.M.M. Vermeulen, M.A. Smits, & J. Haagsma. Production of Apx toxins by field strains of Actinobacillus pleuropneumonia and Actinobacillus suis. Infection and Immunity. 4063-4065. Sept. 1994.
4. Sanford, S.E. Actinobacillus suis: An overview of an emerging disease. Proceedings. American Association of Swine Practitioners. 425-428. 1995.
International Veterinary Pathology Slide Bank: Laser disc frame #5195-6, 9087-8, and 20480.
Dana P. Scott
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-261
DSN: 662-2615
Internet: Scott@email.afip.osd.mil
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.