Results
AFIP Wednesday Slide Conference - No. 14
17 January 1996
- Conference Moderator: Dr. Richard Montali
Diplomate, ACVP
Department of Pathology
National Zoological Park Washington, D.C. 20008
-
- Return to Case Menu
Case I - x5445 (AFIP 2514760)
Signalment: Raccoon (Procyon lotor).
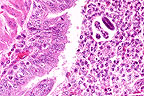 Bronchopneumonia in a raccoon
(Procyon lotor) infected with canine morbillivirus. Note
the prominent intracytoplasmic inclusions within bronchiolar epithelium
and occasionally within macrophages in the bronchiole lumen, and
the presence of metastrongyle larva. (HE, 400X, 122K)
Bronchopneumonia in a raccoon
(Procyon lotor) infected with canine morbillivirus. Note
the prominent intracytoplasmic inclusions within bronchiolar epithelium
and occasionally within macrophages in the bronchiole lumen, and
the presence of metastrongyle larva. (HE, 400X, 122K)
History: This wild raccoon was found standing on a walking
trail in Rock Creek Park by a park visitor around 11:00 A.M.;
the animal did not attempt to flee. The park visitor left the
trail to report the raccoon to the National Park Service (NPS).
A park ranger arrived at the location within the hour and the
raccoon was still standing in the same place. When the ranger
attempted to catch the raccoon, it tried to ascend a nearby tree,
but fell and landed flat on its back. It was then caught without
incident. The raccoon was brought to National Zoologic Park (NZP)
in accordance with an arrangement between NPS and NZP to investigate
suspected distemper-infected wildlife. The animal was euthanized
at NZP, a necropsy was performed and the brain was taken for submission
for rabies testing.
Gross Pathology: The body of this young adult, feral raccoon
is in good nutritional condition with abundant body fat. The eyes
are bilaterally matted with yellow, purulent discharge. The respiratory
tract contains a moderate amount of white, foamy exudate. The
cranial-ventral portion of the right lung lobe is firm, deep red
and consolidated. The stomach contains green, mucous-rich ingesta
and numerous tapeworms in the pylorus which extend into the duodenum.
The remaining small intestine has multifocal areas of hyperemic
mucosa within the jejunum and ileum. More tapeworms are present
within the colon. The luminal contents are creamy green to brown.
The liver and spleen are firm and congested. The right footpad
is thickened and hard. The urinary, musculoskeletal, cardiovascular,
reproductive, endocrine, and CNS systems are unremarkable.
Laboratory Results:
The brain is negative for rabies virus by immunofluorescent
antibody testing.
Contributor's Diagnoses and Comments: 1. Interstitial pneumonia,
giant cell, with intracytoplasmic inclusion bodies, canine distemper
virus. 2. Acute bronchitis with eosinophils, parasitic, Crenosoma
sp.
Lung: Numerous neutrophils fill alveoli and bronchi. There
is abundant necrosis and numerous intraepithelial eosinophilic
cytoplasmic inclusions. There are multiple larval and adult nematode
cross sections within a bronchus, along with associated neutrophils,
eosinophils, macrophages and occasional multinucleated cells.
This is a chronic case of canine distemper. The heavy parasite
load contributed to the clinical disease. Heart blood culture
grew a beta-hemolytic Staphylococcus. Cytology of conjunctival
smears was non-contributory.
AFIP Diagnoses:
1. Lung: Pneumonia, bronchointerstitial, subacute, diffuse, moderate,
with type II pneumocyte hyperplasia, syncytial cells, and eosinophilic
intranuclear and intracytoplasmic inclusion bodies, raccoon (Procyon
lotor), procyonid, etiology consistent with canine distemper
virus.
2. Lung: Bronchitis and bronchiolitis, suppurative and eosinophilic,
multifocal, moderate, with intraluminal adult and larval metastrongyle
nematodes.
Conference Note: Canine distemper virus (CDV) is a morbillivirus
in the Paramyxoviridae family. It causes pneumonia, enteritis
and encephalitis in canines, procyonids, felids, mustelids, hyaenidae,
and viverridae. Clinical signs vary somewhat in the various wildlife
species but are generally similar to those seen in dogs. Attempts
to vaccinate susceptible species with modified-live vaccines have
resulted in vaccine-induced distemper in red pandas, African wild
dogs, black-footed ferrets, maned wolves, grey foxes, bush dogs,
kinkajous, and fennec foxes. Killed vaccines are currently being
developed to protect these exotic and often endangered species.
The pathogenesis in exotic species is presumed to be similar
to that in dogs. In dogs, infection with CDV occurs primarily
by inhalation with the virus localizing in tonsils and bronchial
lymph nodes. After 2-5 days, there is a cell-associated viremia
resulting in infection of lymph nodes, spleen, thymus, bone marrow,
and macrophages in the lamina propria of the stomach and intestine.
At this stage, severe depletion of lymphocytes may develop with
concomitant immunosuppression. At 8-10 days post-infection, the
virus again disseminates, with continued infection of mononuclear
cells and epithelial cells, causing hyperkeratotic dermatitis,
diarrhea, pneumonia, and keratitis. The brain is sometimes affected,
usually after the visceral infection has ended. The virus first
infects macrophages in the meninges and later spreads to ependymal
cells, glial cells, and neurons. Neuronal involvement leads to
behavioral changes and varying degrees of muscular spasm or paresis.
Moribilliviruses possess two proteins which facilitate binding
to host membranes, hemagglutinin and F protein. The F factor mediates
fusion of the viral envelope with the cellular membrane and assists
in viral attachment. It also causes host cell fusion and is responsible
for the formation of syncytial cells. The ability to fuse host
cells allows the virus to spread without being exposed to antibody.
To be biologically active the F protein must be cleaved by a host
protease into two disulfide-linked polypeptides, F1 and F2. If
a host cell lacks the necessary proteases, the virus formed is
not infectious, since the F factor is required for viral attachment.
The intrabronchial metastrongyle was identified by Dr. C.H.
Gardiner, parasitology consultant for the Department of Veterinary
Pathology, as belonging to the genus Crenosoma. Crenosoma vulpis
is a common lungworm of foxes and occurs in other canids and raccoons.
The adults of Crenosoma spp. reside in the bronchi where they
deposit first stage larvae or thin-shelled eggs containing first
stage larvae. The larvae ascend the trachea and are swallowed.
The larvae or eggs pass through the intestinal tract and exit
in the host's feces. They develop into infective third stage larvae
in snails and slugs. The definitive host becomes infected by ingesting
infected gastropods.
Contributor: National Zoological Park, Washington, D.C. 20008
References:
1. Appel, MJG. 1987. Canine Distemper Virus. In, Virus Infections
of Vertebrates, Vol. 1, Virus Infections of Carnivores, Ed. M.
J. Appel, Elseveir Science Publications B.V. pp. 133-159.
2. Appel, MJG, and RJ Montali. 1994. Canine Distemper and Related
Emergent Morbillivirus Disease in Exotic Species. In, Proceedings
of the American Association of Zoo Veterinarians, Pittsburgh.
PA. pp. 336-339.
3. Montali, RJ, CR Bartz, and M Bush. 1987. Canine Distemper
Virus. In, Virus Infections of Vertebrates. Vol. 1 Virus Infections.
4. Georgi, JR, Georgi ME: Parasitology for Veterinarians. 5th
edition, W.B. Saunders Co., Philadelphia, pp. 180-181, 1990.
5. Fenner, FJ, et al: Veterinary Virology. 2nd edition, Academic
Press Inc., San Diego, CA, pg. 471-488, 1993.
International Veterinary Pathology Slide Bank: Laser disc frame
# 13013, 13012, 13011, 6898, 6897, and 4810.
Case II - 95-20060 (AFIP 2503514)
Signalment: Six-month-old male green iguana.
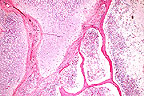 Chondromatosis and osteoporosis
in a green iguana (Iguana iguana)due to nutritional osteodystrophy.
(HE, 400X, 113K)
Chondromatosis and osteoporosis
in a green iguana (Iguana iguana)due to nutritional osteodystrophy.
(HE, 400X, 113K)
History: The iguana was reported to be lethargic and dehydrated
prior to death. The diet consisted solely of fruits and vegetables.
The house had recently been fumigated for insects and the owner
was concerned about the possibility that the iguana was poisoned
by eating a poisoned insect.
Gross Pathology: An immature male green iguana in fair body
condition weighing approximately 70 gm was presented for necropsy.
Both femurs were markedly enlarged in diameter and there was a
pathologic fracture of the distal right femur. Both femurs cut
easily with a scalpel blade. The mandible was rubbery and bent
easily without breaking. Approximately 3.0 mls of serous fluid
was present in the abdominal cavity. The thyroid glands were mildly
enlarged but the parathyroid glands were not observed grossly.
No other significant gross changes were observed in the carcass.
Laboratory Results: Morganella morgani and a Flavobacterium
spp. were isolated from femoral bone. Flavobacterium
spp., Alicaigenes xylosoxidans and Citrobacter freundii
were isolated from an abdominal swab. Flavobacterium spp.
was also isolated from liver. None of these organism were considered
to be significant in this case. Viral culture was not performed.
Blood was not available for hematology and blood chemistry analysis.
Contributor's Diagnosis and Comments: Femur, chondromatosis,
diffuse, severe, bilateral with severe osteoporosis, compatible
with nutritional osteodystrophy.
Sections of undecalcified diaphyseal femur are submitted for
conference participants. The diaphysis of each femurs is markedly
thickened. There is a diffuse lack of cortical bone with a proliferating
cuff of hyperplastic cartilage that has largely replaced and compressed
the diaphyseal cortical bone. Numerous scattered osteoclasts and
a row of osteoblasts line most of the remaining spicules of cortical
bone. Periosteal reaction is minimal. Bone marrow elements are
present in low, but adequate numbers. Sections of mandible (not
submitted) have a diffuse lack of cortical bone with marked diffuse
osteoclastic activity and replacement of bone with a loose areolar
fibrous connective tissue compatible with fibrous osteodystropy
(osteodystrophia fibrosa) . In addition, sections of thyroid gland
exhibited mild to moderate diffuse hypertrophy and hyperplasia
of follicles containing abundant colloid. Parathyroid tissue was
not observed on gross or histologic examination.
The proliferating cuff of diaphyseal hyperplastic cartilage
is unique to the iguana with nutritional osteodystrophy (secondary
nutritional hyperparathyroidism). Fibrous osteodystrophy commonly
occurs in the horse and its relatives (zebra, etc.), goats, pigs,
cattle, sheep (rarely), and a variety of other species, including
non-human primates. Fibrous osteodystrophy is characterized by
extensive osteoclastic resorption of bone and formation of fibro-osseus
tissue which fills the marrow space in response to excessive secretion
of parathyroid hormone. These changes are particularly prominent
in the bones of the face and mandible in some species resulting
in the syndromes of "bighead" (horse) and/or "rubber
jaw" (dog). There is a high susceptibility to pathologic
fractures and avulsion of ligaments resulting from slight trauma.
The most common causes of fibrous osteodystrophy are deficiency
of calcium, calcium/phosphorous imbalance (high dietary phosphorous),
vitamin D deficiency and occasionally renal failure. These conditions
result in excessive secretion of parathyroid hormone. Parathyroid
hormone (PTH) increases resorption of calcium via an osteoblast
mediated stimulation of osteoclasts and decreases resorption of
phosphate from the glomerular filtrate. Osteoclasts do not have
receptors for PTH and only respond to PTH in the presence of osteoblasts
which appear to release unknown paracrine factors that locally
stimulate osteoclastic bone resorption.
AFIP Diagnosis: Femur, cortex: Osteodystrophy, chondroproliferative,
diffuse, severe, with osteopenia, green iguana (Iguana iguana),
reptile.
Conference Note: Captive iguanas are often fed a diet of fruits
and vegetables which contain low levels of calcium. The calcium
deficient diet results in low serum levels of calcium and relatively
high levels of serum phosphorous, inducing the secretion of PTH
from chief cells in the parathyroid. Parathyroid hormone elevates
serum calcium by increasing the active osteoclast pool, resulting
in osteoclasis and skeletal remodeling, and by increasing absorption
of calcium in the distal tubules of the kidney. There is also
a concomitant decrease in serum phosphate due to PTH-mediated
decreases in absorption of phosphates in the proximal tubules
of the kidney. Continued osteoclastic activity in the face of
calcium deficiency results in the lesions associated with osteodystrophy.
It is believed that the cartilaginous proliferation is an adaptation
to mechanical stresses placed upon the weakened bone. It is not
understood why iguanas develop proliferations of cartilaginous
rather than fibrous tissue, as is seen in other species with nutritional
osteodystrophy.
Green iguanas have also been shown to develop fibrous osteodystrophy
if they are not allowed access to ultraviolet light in the range
of 285-315 nm (UV-B). Ultraviolet light is required by iguanas
to convert provitamin D3 to the active form of vitamin D3. It
has been speculated that the hypovitaminosis D causes an exaggerated
parathyroid hormone response resulting in decalcification of bone
and fibrous osteodystrophy. An unusual and characteristic feature
of this condition is widespread metastatic calcification of soft
tissues in the face of hypovitaminosis D.
Contributor: Veterinary Diagnostic and Investigational Laboratory,
College of Veterinary Medicine, University of Georgia, P.O. Box
1389, Tifton, Georgia 31793.
References:
1. Anderson, M.P. and Capen, C.C. 1976. Nutritional osteodystrophy
in captive green iguanas (Iguana iguana). Virchows Arch. B Cell
Pathol. 21:229-247.
2. Anderson, M.P. and Capen, C. C. 1976. Fine structural changes
of bone cells in experimental nutritional osteodystrophy of green
iguanas. Virchows Arch. B Cell Pathol. 20:169-174.
3. Jacobson, E. R. 1981. Diseases of reptiles. Part I. Noninfectious
diseases. Compend. Contin. Educ. Pract. Vet. 3:122-126.
4. Jacobson, E. R. 1984. Biology and diseases of reptiles In:
Laboratory Animal Medicine. Eds. J.G. Fox. , F. J. Cohen and F.
M. Loew. Academic Press, Inc., New York. chap. 15, pp. 470-471.
5. Palmer, N. 1993. Bones and Joints In: Pathology of Domestic
Animals. Eds. K. V. F. Jubb, P. C. Kennedy and N. Palmer. Academic
Press, Inc., new York. Chap. 1, pp. 72-77.
6. Richman L, Montali R, Allan M, and Oftedal O: Widespread
metastatic soft tissue mineralization in vitamin D deficient Green
Iguanas. Abstract, American College of Veterinary Pathologists
46th Annual meeting, 1995.
International Veterinary Pathology Slide Bank: Laser disc frame
#2589, 2590, 6097, 6305, 6309-11, 8223, 9152, 9399, 9981-9988,
15293, 15294, and 15299.
Case III - 424/95 (AFIP 2506142)
Signalment: Thirty-year-old female Asian elephant (Elaphas
maximus).
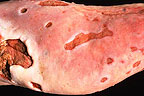 Multifocal
to coalescing lingual ulcers in an Asian elephant. (24K)
Multifocal
to coalescing lingual ulcers in an Asian elephant. (24K)
 Severe
ulcerative pododermatitis and loss of toenaila in an Asian elephant
(70K)
Severe
ulcerative pododermatitis and loss of toenaila in an Asian elephant
(70K)
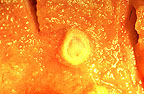 Pox-like ulcerative lesion
on the oral mucosa of an Asian elephant (51K)
Pox-like ulcerative lesion
on the oral mucosa of an Asian elephant (51K)
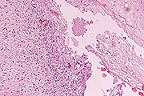 Lingual ulcer in an Asian elephant
(Elaphas maximus) with ballooning degeneration of the adjacent
epithelial cells. Round eosinophilic viral inclusions can be seen
in several epithelial cells. (200X, HE, 96K)
Lingual ulcer in an Asian elephant
(Elaphas maximus) with ballooning degeneration of the adjacent
epithelial cells. Round eosinophilic viral inclusions can be seen
in several epithelial cells. (200X, HE, 96K)
History: This elephant was part of a small circus in Germany.
The animal had a three month history of periodic papular and ulcerative
skin disease. Additional clinical findings included elevated body
temperature and reduced general condition. Poxvirus infection
had been diagnosed, and the animal had been treated symptomatically
without success. After sloughing of the ungula on all four feet
and loss of the solar skin, the elephant was euthanized.
Gross Pathology: At necropsy there were numerous confluent
ulcerative skin erosions (photo) over the entire body. Papular
lesions up to 2.5 cm in diameter were also present but less frequently.
The nails and solar epidermis were completely absent on all four
legs. Similar lesions were detected on the mucosa of the trunk,
the tongue (photo), the larynx and the esophagus. In the proximal
great colon there was an ulcer, 30 cm in diameter, with focal
chronic fibroblastic peritonitis. In the remaining colon numerous
ulcers up to 1 cm in diameter were found. The mesenteric lymph
nodes were necrotic.
Laboratory Results: Cowpox virus was isolated from the skin.
Contributor's Diagnosis and Comments: Glossitis, necrotizing
and ulcerative, diffuse, severe with bacterial colonies on the
necrotic surface, ballooning degeneration in the neighboring epithelium
and occasionally intracytoplasmic, amphophilic to eosinophilic
inclusions. Etiology: cowpox virus.
Poxvirus infections in zoo-kept animals are restricted to Central
Europe. Twenty-four of twenty-six reported outbreaks between 1960
and 1990 were localized in a 1070 km diameter circle with a center
near Magdeburg, Germany. Only a single outbreak in Moscow occurred
outside this circle. The infection has been observed in rhinoceroses,
okapis and other mammals, including felines. In elephants (Asian
and African) 78 cases of poxvirus infection have been registered.
In 9 cases the disease was fatal. The isolated virus strains were
identified as cowpox virus.
The occurrence of these outbreaks and the restriction to a
limited region within Europe (the infection does not occur in
the natural habitats of these species) support the suspicion that
zoo-kept animals are only an indicator of a hidden virus cycle
that involves a local feral mammal. The high prevalence of antibody
titers against cowpox virus in wild rodents indicate a possible
role of these species in the transmission of the infection.
The present case is a very dramatic one. The long treatment
was justified by the cooperative behavior of the animal as well
as by social and economical arguments. The severity and extensiveness
of the lesions are probably due to the long survival and relapsing
viremias.
AFIP Diagnosis: Tongue: Glossitis, ulcerative, subacute, focally
extensive, severe, with ballooning degeneration and eosinophilic
intracytoplasmic inclusion bodies, Asian elephant (Elaphas
maximus), proboscid.
Conference Note: After a pox virus was identified as the causative
agent in the European zoo animal outbreaks, children who had recently
been vaccinated against smallpox and were allowed to ride the
zoo elephants were suspected to have transferred the live virus
to the elephants. Attempts to reproduce the disease with vaccinia
virus strains have been unsuccessful. Polypeptide analysis has
demonstrated that the elephant strains of virus and ectromelia
virus produce the same polypeptide pattern, each lacking a 53,000
kd molecular weight polypeptide that is present in vaccinia. The
elephant virus strains also lack a polypeptide of 37,000 kd that
is present in cowpox. In addition, DNA cleavage patterns of the
virus strains isolated from zoo-kept mammals in Europe show a
high degree of similarity and can be distinguished from cowpox
and vaccinia virus, although some genetic patterns are shared.
Since the elephant virus resembles cowpox, this virus is now known
as a cowpox-like virus.
The elephant cowpox-like virus has also been isolated from
a human in the Netherlands who has had no contact with zoo animals,
but was in close contact with many cats, a rabbit, a guinea pig,
and a dog. The virus was also isolated from a cat in the Netherlands.
These findings suggest that the reservoir may be a small mammal
hunted by cats.
Other orthopoxviruses of importance in man and animals are
vaccinia virus, ectromelia virus, monkeypox virus, camelpox virus,
Uasin Gishu disease virus, Tatera poxvirus, raccoon poxvirus,
vole poxvirus, and seal poxvirus.
Contributor: Institut f
r Veterin„r-Pathologie, Frankfurter Strasse 96, D-35392,
Geissen.
References:
1. Pilaski J and R”sen-Wolff A: Poxvirus infection in zoo-kept
mammals, in Virus diseases in laboratory and captive animals,
Darai G (ed), Martinus Nijhoff Publishers, Boston, 83 - 100, (1988).
2. Pilaski J and Jacoby F: Die Kuhpocken-Erkrankungen der Zootier,
Verh.Ber.Erkrg.Zootier, 35: 39 - 50, (1993).
3. Pade K, R
edi D, Pilaski J, Heldstab A and M
ller M: Ein verlustreicher Pockenausbruch bei asiatischen Elefanten
(elaphas maximus) in einem deutschen Wanderzirkus, Verh.ber.Erkrg.Zootier,
32: 147 - 155, (1990).
Case IV - 950581-8 (AFIP 2507590)
Signalment: Australian parrot (Barnardius sp.)
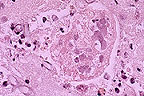 Syncytial cell with numerous
intranuclear inclusions in the lung of an Australian parrot with
tracheobronchitis. Negatively stained outlines of fungal hyphae
can be seen in the adjacent tissues. (HE, 400X, 76K).
Syncytial cell with numerous
intranuclear inclusions in the lung of an Australian parrot with
tracheobronchitis. Negatively stained outlines of fungal hyphae
can be seen in the adjacent tissues. (HE, 400X, 76K).
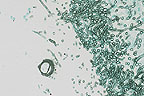 Fruiting body and fungal hyphae
of Aspergillus from the lung of an Australian parrot with
necrotizing tracheobronchitis. (GMS, 400X, 43K)
Fruiting body and fungal hyphae
of Aspergillus from the lung of an Australian parrot with
necrotizing tracheobronchitis. (GMS, 400X, 43K)
History: This Australian parrot was presented for necropsy
examination with a history of respiratory difficulties and sneezing.
Gross Pathology: Gross lesions were confined to the respiratory
system, and consisted of a fibrinous tracheitis throughout the
trachea and a necrotic tracheobronchitis at the bronchial bifurcation
characterized by a blackish discoloration and presence of hairy
mycelial elements in the bronchial lumen.
Laboratory Results: None submitted.
Contributor's Comments:
Contributors diagnosis: Necrotic bronchitis with syncytial
cells and intranuclear acidophilic herpesvirus inclusions and
a more severe necrotic bronchitis due to Aspergillus sp.
infection.
Microscopic lesions are confined to the respiratory system.
There are two lesions of different origin:
1. A severe necrotic bronchitis with tangles of hyphae and
conidiospores in the bronchial lumen. These fungal elements invade
the surrounding parenchyma and the blood vessels.
2. A necrotic viral bronchitis characterized by the presence
of syncytial cells and acidophilic intranuclear inclusions in
the bronchial epithelium similar to that of avian laryngotracheitis.
A similar case of severe respiratory herpes virus infection in
parrots was reported.
AFIP Diagnoses:
1. Lung: Bronchopneumonia, necrotizing and granulomatous, focally
extensive, moderate, with necrotizing vasculitis and numerous
fungal hyphae, Australian parrot (Barnardius sp.), avian.
2. Lung: Syncytial cells, numerous, with eosinophilic intranuclear
inclusion bodies.
Conference Note: A herpesvirus serologically related to infectious
laryngotracheitis has been identified in Amazon and Bourke's parrots.
Clinically affected parrots develop fibronecrotic ocular, nasal,
and/or oral discharges accompanied by open mouth breathing and
coughing. Histologically there is necrosis of respiratory epithelium
and a resultant fibrinonecrotic bronchopneumonia. Birds frequently
develop secondary fungal and bacterial infections and often die
from asphyxiation caused by obstruction of the trachea by necrotic
debris.
We have also seen uncomplicated cases of herpesviral infection
in parrots that were characterized by pulmonary synctial cells
formation and epithelial intranuclear inclusion bodies in the
absence of an inflammatory response.
Aspergillus spp. are ubiquitous saprophytic molds. Hyphae of
Aspergillus spp. are 3 to 6 æm in width, are septate,
have parallel walls, and demonstrate dichotomous branching. The
conidial heads are distinctive and are composed of a terminal
vesicle with one or two layers of phialides which produce chains
of conidia, (also called conidiospores), from their tips. The
vesicle merges at its base with a conidiophore (the section of
hyphae that supports the conidial head).
Pulmonary infections with Aspergillus center on bronchi
and bronchioles. Histologically the bronchioles are effaced by
a granulomatous inflammatory infiltrate and necrotic debris. Masses
of tangled fungal hyphae are usually apparent within the bronchiolar
lumen and surrounding parenchyma. After colonization in the lung,
Aspergillus can invade pulmonary arteries or arterioles, resulting
in occlusion of the vessel and formation of a lobular infarct.
In most cases, there are multiple lobular pulmonary infarcts which
frequently coalesce. Large areas of infarction and proliferating
fungal hyphae often cavitate. Hematogenous spread of the fungi
often follows vascular invasion, commonly producing lesions in
the central nervous system, myocardium, liver, and spleen.
Contributor: Laboratoire d'Anatomie Pathologique 7, Avenue
du G‚nŠral de Gaulle 94704 MAISONS ALFORT - FRANCE.
References:
1. Wintroll, G and Gylstorff, L; Schwere durch Herpesvirus verursachte
Erkrankung des respirationsapparates bei Amazonen. 1979; Berline-und-Muchener-Tierarztliche-Wochenschrift.
92, 14, 277-280.
2. Chandler FW and Watts JC: Pathologic diagnosis of fungal
infections. ASCP Press, Chicago, pp. 55-74, 1987.
3. Gerlock H: Viruses in Avian Medicine: Principles and Applications.
Richie BW, Harrison GJ, and Harrison LR eds., Wingers Publ., Florida,
pp. 874-885, 1994.
International Veterinary Pathology Slide Bank: Laser disc frame
#1174-75, 2875, 3484, 3486, 3494, 9291, 11074, 11143, 11297, 11298-99,
13863, 19384, 19473-74, 19495-97, 19517-18, 20546, 20547-50, 24059-62,
and 24667-69.
Dana P. Scott
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: Scott@email.afip.osd.mil
* The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
 Bronchopneumonia in a raccoon
(Procyon lotor) infected with canine morbillivirus. Note
the prominent intracytoplasmic inclusions within bronchiolar epithelium
and occasionally within macrophages in the bronchiole lumen, and
the presence of metastrongyle larva. (HE, 400X, 122K)
Bronchopneumonia in a raccoon
(Procyon lotor) infected with canine morbillivirus. Note
the prominent intracytoplasmic inclusions within bronchiolar epithelium
and occasionally within macrophages in the bronchiole lumen, and
the presence of metastrongyle larva. (HE, 400X, 122K) Chondromatosis and osteoporosis
in a green iguana (Iguana iguana)due to nutritional osteodystrophy.
(HE, 400X, 113K)
Chondromatosis and osteoporosis
in a green iguana (Iguana iguana)due to nutritional osteodystrophy.
(HE, 400X, 113K) Multifocal
to coalescing lingual ulcers in an Asian elephant. (24K)
Multifocal
to coalescing lingual ulcers in an Asian elephant. (24K) Severe
ulcerative pododermatitis and loss of toenaila in an Asian elephant
(70K)
Severe
ulcerative pododermatitis and loss of toenaila in an Asian elephant
(70K) Pox-like ulcerative lesion
on the oral mucosa of an Asian elephant (51K)
Pox-like ulcerative lesion
on the oral mucosa of an Asian elephant (51K) Lingual ulcer in an Asian elephant
(Elaphas maximus) with ballooning degeneration of the adjacent
epithelial cells. Round eosinophilic viral inclusions can be seen
in several epithelial cells. (200X, HE, 96K)
Lingual ulcer in an Asian elephant
(Elaphas maximus) with ballooning degeneration of the adjacent
epithelial cells. Round eosinophilic viral inclusions can be seen
in several epithelial cells. (200X, HE, 96K) Syncytial cell with numerous
intranuclear inclusions in the lung of an Australian parrot with
tracheobronchitis. Negatively stained outlines of fungal hyphae
can be seen in the adjacent tissues. (HE, 400X, 76K).
Syncytial cell with numerous
intranuclear inclusions in the lung of an Australian parrot with
tracheobronchitis. Negatively stained outlines of fungal hyphae
can be seen in the adjacent tissues. (HE, 400X, 76K). Fruiting body and fungal hyphae
of Aspergillus from the lung of an Australian parrot with
necrotizing tracheobronchitis. (GMS, 400X, 43K)
Fruiting body and fungal hyphae
of Aspergillus from the lung of an Australian parrot with
necrotizing tracheobronchitis. (GMS, 400X, 43K)