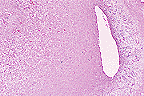
 Unilateral uterine growth in
an aged female F344 rat. (10X, HE, 101K)
Unilateral uterine growth in
an aged female F344 rat. (10X, HE, 101K)Signalment: 10-month-old female F344 rat
 Unilateral uterine growth in
an aged female F344 rat. (10X, HE, 101K)
Unilateral uterine growth in
an aged female F344 rat. (10X, HE, 101K)
History: Incidental finding in an aging rat study.
Gross Pathology: Unilateral enlargement of uterine horn.
Laboratory Results: None submitted
Contributor's Diagnosis and Comments: Female F344 rat: Uterine horn, deciduoma.
AFIP Diagnosis: Uterus: Deciduoma, F344 rat, rodent.
Conference Note: Deciduomas are non-neoplastic proliferations that mimic normal decidual implantation sites. Deciduomas may be focal or multiple, unilateral or bilateral. This proliferative reaction has a limited course that ends with degeneration of the decidual cells. Formation of deciduomas is dependant upon non-specific stimuli following uterine exposure to progesterone for at least 48 hours followed by small amounts of estrogen. Stimuli known to induce deciduoma formation include electrical stimulation, endometrial trauma, and intrauterine injection of salt solutions, oily fluids, and air.
Deciduomas are highly structured and consist of a metrial gland located in the myometrium on the mesometrial side of the uterus, a narrow basal zone, a capsule immediately adjacent to the inner border of the basal zone, a mesometrial region, an antimesometrial region and a transitional area between the mesometrial and antimesometrial regions.
The basal zone is formed from the compressed remnant of endometrial stroma and glands. The metrial gland consists of large round cells with abundant granular cytoplasm and PAS-positive intracytoplasmic granules. The mesometrial region is composed of cells similar to the cells of the metrial gland and cells known as spiny cells which are occasionally binucleate, have long cytoplasmic processes and abundant glycogen. The cells of the antimesometrial region are closely packed with abundant
eosinophilic to amphophilic cytoplasm and irregularly round, vesiculate nuclei. The transitional zone is composed of loosely arranged cells resembling the spiny mesometrial cells.
Contributor: Pathology Associates, Inc., 15 Worman's Mill Court, Frederick, MD 21701.
Reference: Boorman GA, et al (eds): Pathology of the Fischer Rat, pages 450-452, Academic Press, 1990.
Case II - LM 52 (AFIP 2506270)
Signalment: Male B6C3F1 mouse approximately 790 days old.
History: Part of the low dose group of a two year (735 days) dermal bioassay of a detergent component. The mouse was killed at the termination of the study. No clinical abnormalities were noted.
Gross Pathology: The liver had an irregular surface and multiple protuberant brown nodules (up to 3 x 3 x 3 mm).
Laboratory Results: None available.
Contributor's Diagnosis and Comments: Chronic-active hepatitis with oval cell proliferation, bile duct hyperplasia, and regenerative hepatocellular hyperplasia. Helicobacter hepaticus infection.
There is bile duct and oval cell proliferation, variably severe lymphocytic cholangitis, marked nodular hepatocellular hyperplasia, minimal periportal fibrosis, and scattered sites of hepatocellular necrosis. The liver surface is irregular - depressed where there is loss of hepatocytes and/or fibrotic replacement, and/or elevated by hepatocellular proliferative lesions (foci, regenerative hyperplasia). There are focal aggregates of proliferated bile ducts in all slides; some slides also have aggregates of rounded empty spaces lined by flattened or plump endothelium. Oval cells morphologically similar to bile ductule epithelium are frequent in sites of periportal fibrosis or adjacent to proliferated bile ductules. In many slides there is a protruding hepatocellular proliferative lesion that has marked angiectasis.
Steiner's modification of the Warthin-Starry stain demonstrated helical bacteria between hepatocytes in many mice in this study. Lesions and bacteria were most frequent in male mice and were in all treatment groups. These bacteria (Helicobacter hepaticus), located within bile canaliculi, and these typical lesions, have recently been characterized and are associated with a high incidence of hepatocellular neoplasms (1,2). Another organism, Helicobacter bilis, has also been found in the livers of aged mice, often along with Helicobacter hepaticus (3). A hepatotoxic factor has been associated with Helicobacter hepaticus and other Helicobacter species (4). There are other reports of an association between liver necrosis in rodents and subsequent hepatocellular proliferation and neoplasia (5).
AFIP Diagnosis: Liver: Hepatitis, lymphoplasmacytic, chronic, portal and periportal, with hepatocellular necrosis, biliary hyperplasia, regenerative hepatocellular hyperplasia, and neutrophilic cholangitis, B6C3F1 mouse, rodent.
Conference Note: This case was reviewed in consultation with AFIP's Department of Hepatic Pathology. The term chronic-active hepatitis is being replaced in the human literature by chronic hepatitis. The hallmark of chronic hepatitis is the presence of chronic periportal inflammation and piecemeal necrosis. Piecemeal necrosis refers to a periportal infiltration of lymphocytes with necrosis that destroys the limiting plate of hepatocytes, and is followed by fibrosis. The degenerating hepatocytes and lymphocytes are closely apposed, the lymphocytes often being located within the space of Disse. Occasionally the lymphocytes indent the cytoplasm of the hepatocytes (polesis) or the hepatocyte may completely encircle the lymphocyte (emperipolesis). Another highly characteristic feature of chronic hepatitis in humans is the entrapment of single or groups of hepatocytes within expanded portal areas.
The liver sections from the mouse in this case display some of the characteristics described for chronic hepatitis; however, the lack of piecemeal necrosis distinguishes this lesion from the human condition. Also present are areas of angiectasis that are primarily limited to regenerative nodules of hepatocytes. The pathogenesis of this vascular lesion is not known; we hypothesize that it may be related to disturbances in blood flow secondary to compression caused by regenerative hepatic nodules. Spiral bacteria within bile ducts stain with the Warthin-Starry technique and demonstrate immunoreactivity after immunohistochemical procedures for H. pylori, which has been reported to cross react with H. hepaticus.
Helicobacter spp. are gram-negative, 3.5 æm x 1.0 æm, S-shaped or spiral rods. The following are Helicobacter spp. that have been associated with disease: Helicobacter mustelae (a cause of chronic gastritis in ferrets), Helicobacter felis (a cause of gastritis in dogs and cats), and Helicobacter pylori (which is associated with chronic gastritis in man and primates and peptic ulcer disease in man).
The pathogenesis of Helicobacter infection in not fully understood; however, it has recently been determined that Helicobacter hepaticus (as well as H. pylori, H. felis, and H. mustelae) produces a cytotoxin that induces a cytopathic effect in mouse liver cell lines. It is likely that this cytotoxin is involved in the formation of the hepatic lesions in mice and gastric lesions in other animals.
Researchers have noted a higher incidence of hepatocellular tumors in mice infected with H. hepaticus, suggesting that the bacteria may be associated with the development of the tumors. A similar relationship has been suggested between the formation of human gastric adenocarcinoma and H. pylori. Mice with chronic hepatitis caused by H. hepaticus may serve as an animal model for investigation into the association of chronic bacterial infection and the development of neoplasia.
The differential diagnosis for hepatic necrosis in the mouse includes mouse hepatitis virus, Tyzzer's disease, ectromelia virus, Clostridium piliforme, Salmonella enteritidis, and various hepatotoxins.
Contributor: National Institute of Environmental Health Sciences, P.O. Box 12233, Research Triangle Park, NC 27709.
References:
Signalment: 11-month-old male Sprague-Dawley rat
History: Rat was part of a breeding colony. Mass detected in abdominal area.
Gross Pathology: 0.6 x 3 x 3 cm subcutaneous mass present, left abdominal region.
Laboratory Results: None submitted
Contributor's Diagnosis and Comments: Preputial gland adenoma.
This is not a commonly observed neoplasm in the rat. The preputial gland neoplasms in males (clitoral gland in females) are rarely malignant. This neoplasm if left untreated would be expected to enlarge without marked local invasion or metastasis.
AFIP Diagnosis: 1. Haired skin, preputial gland: Preputial
gland adenoma, Sprague-Dawley rat, rodent.
2. Haired skin, preputial gland: Adenitis, lymphoplasmacytic,
chronic, multifocal, moderate.
Conference Note: Preputial glands and clitoral glands are compound holocrine, branched tubuloalveolar glands derived from sebaceous glands. Preputial and clitoral gland tumors are histologically identical, and their biological behavior is the same. Both preputial and clitoral gland adenomas are usually small and difficult to detect grossly. Larger tumors are prone to trauma and ulceration due to their location on the ventral surface of the animal.
Microscopically, the preputial gland adenoma is a well-circumscribed, expansile multilobulated mass composed of neoplastic polygonal cells arranged in acini, clusters, cords and lobules separated by a fine fibrovascular stroma. The neoplastic cells have distinct cell borders with a moderate amount of eosinophilic granular cytoplasm. Neoplastic cells located in the center of the lobules contain many intensely eosinophilic intracytoplasmic granules. The nuclei are irregularly round with finely stippled chromatin and a distinct nucleolus. The mitotic rate is less than one per ten high power fields. Central necrosis of neoplastic cells may be present in lobules.
Preputial gland adenomas can be difficult to differentiate from preputial gland adenocarcinomas. Adenocarcinomas are usually less differentiated; loss of glandular growth pattern, increased cellular atypia and infiltration are usually seen. The differential diagnosis also includes mammary gland and sebaceous gland neoplasms.
Contributor: Roche Bioscience, 3401 Hillview Avenue, R2-222, Palo Alto, CA 94303.
Reference: Parker GA, and Grabau J: Adenoma and adenocarcinoma, preputial gland, rat. In: Jones TC, Mohr U, and Hunt RD (Eds). Genital System, Monographs on Pathology of laboratory Animals. Springer-Verlag, Berlin 1987; p 275-281.
Signalment: Imported young adult male cynomolgus monkey (Macaca fascicularis).
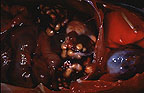 Numerous variably-sized hepatic
granulomas in a cynomolgus monkey (34K).
Numerous variably-sized hepatic
granulomas in a cynomolgus monkey (34K).
History: No medical problems were noted during quarantine, and 3 consecutive tuberculin tests were negative. After the animal had been in the facility for approximately 4 months, a draining skin wound over the back was discovered. The wound was examined, cleaned, and treated topically, and the condition appeared to resolve. When the animal was examined approximately one month later during routine tuberculin testing, several small draining tracts were found over the back near the area of the original wound. Systemic antibiotic therapy was initiated. The tuberculin test was negative. The monkey was found dead in its cage five days after tuberculin administration.
Gross Pathology: White, firm to gritty, nodular masses 2-20 mm in diameter were found in the lung, liver, spleen, tracheobronchial lymph nodes, and sublumbar lymph nodes. These masses contained white, solid, caseous centers. There were adhesions of loops of intestine to the root of the mesentery.
Laboratory Results: Staphylococcus aureus was isolated from cultures of the draining skin lesions.
Contributor's Diagnosis and Comments: Hepatitis, granulomatous, multifocal, with necrosis, multinucleated giant cells, and few intracellular acid-fast bacilli (tuberculosis).
Granulomatous lesions in lungs, liver, spleen, lymph nodes, and colonic gut-associated lymphoid tissue contained areas of caseous necrosis, with occasional foci of mineralized necrotic debris. Few acid-fast organisms were detected, which is common in cases of simian tuberculosis. This animal had at least 5 negative tuberculin tests. Cynomolgus monkeys often respond poorly to tuberculin challenge, and may carry lesions of tuberculosis without evidence of clinical disease. In this monkey, abdominal lesions were more numerous and larger than pulmonary lesions.
AFIP Diagnosis: Liver: Granulomas, multiple, with bridging portal fibrosis and biliary hyperplasia, cynomolgus monkey (Macaca fascicularis), non-human primate.
Conference Note: The most common agents of tuberculosis in primates are Mycobacterium tuberculosis and M. bovis. Organisms in the M. avium-intracellulare group are frequently isolated in macaques with mycobacterial infections in which tubercle formation is not a feature.
Mycobacterium tuberculosis has no known endotoxin or exotoxins. Due to the presence of several compounds in their cell wall, mycobacteria are able to escape killing by phagocytic cells and induce delayed hypersensitivity. Cord factor is a surface glycolipid in the cell wall of M. tuberculosis. Absent from nonpathogenic mycobacteria, cord factor is known to stimulate granuloma formation. Sulfatides are sulfur containing glycolipids in the cell wall of mycobacteria that prevent fusion of phagosomes of macrophages containing M. tuberculosis with lysosomes. Another cell wall constituent, LAM, is a heteropolysaccharide similar to the endotoxins of gram-negative bacteria. LAM inhibits macrophage activation by interferon-gamma and induces macrophages to secrete TNF-à and IL-10 which suppresses mycobacteria-induced T-cell proliferation.
The mycobacteria are initially able to replicate in naive macrophages. After a few weeks, however, a T-cell mediated immunity develops (delayed hypersensitivity). CD4+ helper T cells activate macrophages by secreting interferon-gamma, enabling the macrophages to kill the bacilli via the release of reactive nitrogen intermediates. CD8+ suppressor T cells kill macrophages that harbor mycobacteria, causing caseous necrosis; the mycobacteria cannot grow in the acidic, extracellular environment of the caseous core of a granuloma. The classic granuloma of tuberculosis is the result of this process.
The disseminated disease seen in this case indicates that the bacterial strain was highly virulent or that the monkey was particularly susceptible. Mycobacteria can spread hematogenously to many organs when granulomas rupture into blood vessels. The granulomas may also rupture into airways, allowing the mycobacteria to be released and transmitted to other hosts in aerosols.
It is speculated that false negative tuberculin tests (anergy) result from overwhelming infections or immunodeficiency, possibly due to a loss of CD4+ T-cells. Cynomolgus macaques tend to react erratically to both the purified protein derivative (PPD) and mammalian old tuberculin (MOT) intradermal tests. Research is being done to develop enzyme-linked immunosorbent assays and polymerase chain reaction tests to accurately and rapidly identify and diagnosis mycobacterial infections in man and animals.
Contributor: Abbott Laboratories, Department of Pathology, D-469, AP13A, 100 Abbott Park Road, Abbott Park, IL 60064-3500.
References:
International Veterinary Pathology Slide Bank: Laser disc frame #24, 22650, 22651, and 22652.
Dana P. Scott
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: Scott@email.afip.osd.mil
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.