JPC SYSTEMIC PATHOLOGY
DIGESTIVE SYSTEM
September 2021
D-P01
SLIDE A: Signalment (JPC #1925258): A 2-month-old chicken.
HISTORY: None.
HISTOPATHOLOGIC DESCRIPTION: Cecum: Circumferentially expanding the lamina propria and infiltrating the mucosal/crypt epithelium are abundant apicomplexan coccidians at varying developmental life stages including numerous intracellular macrogamonts and microgamonts, intra- and extracellular schizonts, and developing oocysts. Macrogamonts are 10-20 um in diameter with a single, central nucleus and a peripheral ring of 2 um diameter eosinophilic granules. Microgamonts are round to oval, 15-20 um in diameter, filled with multiple 1um basophilic nuclei. Schizonts are 40-60 um in diameter filled with numerous basophilic, 4x2um, crescentic merozoites. Oocysts are within the crypt epithelium or crypt lumina, are 15-20um diameter, thick walled, oval to irregular, and filled with eosinophilic pale granular material and/or a single eosinophilic central nucleus, admixed with a small amount of necrotic debris. Multifocally, the mucosa is eroded with loss of enterocytes and replacement by hemorrhage, fibrin, edema, and eosinophilic cellular and karyorrhectic debris, few lymphocytes and heterophils. Expanding the lamina propria and submucosa and extending into the muscularis and serosa is a moderate inflammatory infiltrate composed of lymphocytes, plasma cells, macrophages, and few heterophils admixed with hemorrhage, fibrin, and edema. The cecal lumen is distended with numerous coccidians as previously described, fibrin, hemorrhage, edema, necrotic debris, degenerate inflammatory cells, sloughed enterocytes, and few clusters of cocci and bacilli.
MORPHOLOGIC DIAGNOSIS: Cecum: Typhlitis, erosive and lymphoplasmacytic, diffuse, marked, subacute, with abundant intra and extracellular coccidian gamonts, schizonts, and oocysts.
CAUSE: Eimeria tenella
SLIDE B: Signalment (JPC #4017836-00): Adult female intact alpaca.
HISTORY: Four days after introduction into new herd, patient began having watery diarrhea, neurologic signs, drooping of lower lip, and open mouth breathing, then died.
HISTOPATHOLOGIC DESCRIPTION: Small intestine: Circumferentially expanding the lamina propria and infiltrating the mucosal and crypt epithelium are abundant developing apicomplexan coccidial life stages including macrogamonts, microgamonts, schizonts, and oocysts. Macrogamonts are 80-100um in diameter and contain peripheralized, brightly eosinophilic granules with an occasional round, central nucleus. Microgamonts are 70-80um in diameter and contain multiple 1um basophilic nuclei. Schizonts are 150-200 um in diameter and contain nuclei arranged in circular blastophores (megaloschizont). Oocysts are within the crypt epithelium or crypt lumina and are 15-20 um in diameter, oval to irregular, and filled with eosinophilic, pale, granular material. Multifocally the villar enterocytes are lost and villi are blunted, fused, eroded, and replaced with rare hemorrhage, fibrin, cellular debris, lymphocytes, plasma cells, and macrophages. The lamina propria, submucosa, muscularis, and serosa are mildly expanded by few lymphocytes and plasma cells.
MORPHOLOGIC DIAGNOSIS: Small intestine: Enteritis, lymphoplasmacytic, diffuse, moderate, subacute, with villous blunting and loss and abundant intra- and extracellular coccidian gamonts, schizonts, and oocysts.
CAUSE: Eimeria macusaniensis
ETIOLOGIC DIAGNOSIS: Cecal/enteric coccidiosis (Eimeriosis)
GENERAL DISCUSSION:
- Phylum Apicomplexa: Intracellular parasites characterized by a life cycle stage with a typical “apical complex” of organelles at one end of the organism
- Highly host specific, and specific to the segment of bowel they infect; direct life cycle; grow and multiply intracellularly in epithelial and subepithelial cells in the alimentary tract
- Chickens:
- Important worldwide poultry disease; causes enteritis and/or typhlitis
- Coccidiosis typically affects intensively managed animals; usually mild disease, severe outbreaks may have high mortality
- Eimeriosis is a disease of young animals, occurring in chicks 3 to 6 weeks of age ( necatrix infects chicks at 8 to 18 weeks)
- Immunity is species specific; experimentally, resistance to coccidial infection is largely from T-cell promoted intracellular killing, directed primarily at asexual stages
PATHOGENESIS:
- Oral ingestion > enterocytes are damaged when mature schizonts rupture and release merozoites
- Malabsorption due to villus atrophy
- Exudative enteritis and typhlitis due to epithelial erosion and ulceration
- In alpacas, infection with macusaniensis can predispose to other infections, especially clostridial infections, due to destruction of intestinal crypts
LIFE CYCLE:
- Endogenous stages are all intracellular, except merozoite and microgamete
- Sporulated oocysts ingested > excystation of oocysts via enzymatic degradation in duodenum > release of sporozoites> free sporozoites invade mucosal epithelium > sporozoites become trophozoites > trophozoites divide to become schizonts (a large encapsulated structure containing many merozoites) > 2-3 generations of schizogony (asexual multiplication) > gametogony (sexual reproduction): schizonts and epithelial cells rupture and release merozoites > merozoites invade adjacent epithelial cells and form either macrogametocytes (female; unicellular; fill the parasitized cell) or microgametocytes (males; undergo multiple divisions and form flagellated microgametes) > fertilization of macrogamete by microgamete, formation of oocyst > unsporulated oocyst excreted > oocyst sporulates in environment (sporogony)
- Sporogony: Outside the host, the unsporulated oocyst undergoes meiosis to produce sporozoites; occurs in warm, moist environment
- The number of reproductive cycles is fixed (in a given species) and unidirectional; barring reinfection, coccidia are self-limiting
TYPICAL CLINICAL FINDINGS:
Chickens:
- Bloody diarrhea
- Bleeding, weight loss, loss of skin pigment
Alpacas:
- Lethargy, weight loss, anorexia, diarrhea, sudden death
TYPICAL GROSS FINDINGS:
Chickens:
- Varies from excessive fluid in intestinal lumen to intestinal dilation and gray-yellow foci visible on the serosal surface; fibrinonecrotic enteritis in severe cases
- tenella (cecal coccidiosis): Ceca filled with caseous cores (dry compact white mass) mixed with blood; thickened cecal wall (edema and cellular infiltrates); petechiae visible from serosal surface, sometimes white spots; comb and visceral organs pale from blood loss; high mortality; blood in droppings
- necatrix: Mid-intestine (jejunum and ileum) markedly distended with yellow to orange mucus; red and white (clumps of schizonts) foci; ballooning intestinal walls; dysentery; petechiae and white spots visible from serosal surface; blood in droppings
- maxima: Mid-intestine (jejunum and ileum, +/- duodenum) mildly distended with excessive orange mucus or distended with fluid and thick bloody mucus covering mucosa; discrete focal hemorrhagic lesions on mucosal surface; soft mucoid salmon-pink colored feces; largest species, with a golden brown oocyst wall
- acervulina: Duodenum +/- upper jejunum with white irregular linear lesions (zebra striping or ladder rung) to coalescing white plaques associated with gamonts and oocysts clustered in villi; hemorrhage is NOT a feature
Alpacas:
- Jejunum and ileum are typically affected, colon and duodenum are rarely affected
TYPICAL LIGHT MICROSCOPIC FINDINGS:
- Organisms in various stages of development randomly scattered within enterocytes or free in the lamina propria
- Oocysts: 15um ( tenella) or 100x80um (E. macusaniensis), thick walled, oval to irregular, filled with eosinophilic pale granular material and/or a single eosinophilic central nucleus
- Asexual replication: Schizogony or merogony
- Schizonts: Asexual replication/schizogony or merogony; encapsulated cyst containing cresentic 2x5um merozoites
- Sexual replication: Gametogony
- Macrogamont (pink females): Sexual replication/gametogeny; 40um ( tenella) or >100um* (E. macusaniensis), round with a central nucleus and 2um peripheral eosinophilic granules
- Microgamont (blue males): Sexual replication/gametogeny; 30um ( tenella) or >100um* (E. macusaniensis) round with numerous basophilic nuclei (microgametes)
- * macusaniensis is by far the largest camelid coccidian
- Hemorrhage and necrosis near blood vessels of the inner circular layer of the tunica muscularis (early)
- Lamina propria, submucosa: Lymphocytic, heterophilic, and eosinophilic infiltrates
- Destruction of the mucosa and tunica muscularis; disrupted cecal glands (chicken)
- Sloughed parasitized enterocytes, blood, and necrotic debris within the intestinal lumen, glands and crypts
- macusaniensis may efface the jejunal and ileal paerenchyma; villar necrosis and loss are the primary lesions, although lamina propria fibrosis may occur with chronicity; coccidians are typically present most densely in the deep crypts but can affect the entire villus in severe infections
ULTRASTRUCTURAL FINDINGS:
- Schizont: Present within a parasitophorous vacuole; single walled; contains cisternae of the parasitic endoplasmic reticulum; free ribosomes; mitochondria; multiple merozoites
- Merozoites: Crescent shaped; have an inner and outer membrane; conoid apparatus; micronemes; rhoptries; nuclei; parasitic endoplasmic reticulum and free ribosomes; may be present within the parasitophorous vacuole
ADDITIONAL DIAGNOTIC TESTS:
- Fecal flotation; histopathology; gross pathology; IFA, PCR
- In alpacas with macusaniensis, fecal flotation is usually negative in early infection even with heavy infection
DIFFERENTIAL DIAGNOSIS:
Chickens:
Necrotizing typhlitis:
- Salmonella enteriditis: Can cause necrotizing enteritis and typhlitis; not specific to one section of the intestine
- Histomonas meleagridis (blackhead): Turkey poults, game birds, chickens, replacement pullets; cecal swollen with cecal cores; circular or oval recessed lesions in liver (pathognomonic lesions)
Enteritis:
- Clostridium perfringens: Chickens; focal or diffuse necrosis of intestinal mucosa, especially ileum; common in the presence of coccidiosis
- Clostridium colinum (quail disease): Captive game birds, turkeys, chickens; acute enteritis only; deep ulcers along intestine; enlarged spleen; focal and/or diffuse yellow areas in liver; often secondary in chickens
- Nonspecific enteritis: Poultry and other birds; enteritis accompanies many infectious diseases that have lesions of greater diagnostic value in other systems (cholera, erysipelas, vibrionic hepatitis, spirochetosis, botulism, aflatoxicosis; influenza, candidiasis, and others)
Alpacas (and other new world camelids):
- Other intestinal Eimeria : E. lamae, E. alpacae, E. punoensis, E. ivitaensis; these are all typically a quarter of the size of E. macusaniensis, and none cause as severe clinical signs
- Cryptosporidia: 4-5um diameter, basophilic, round organisms along the apical surface of enterocytes (intracellular but extracytoplasmic); most dense on the tips of small intestinal epithelium; villar atrophy is the main lesion
COMPARATIVE PATHOLOGY:
|
Animal |
Coccidia |
Organ affected/Clinical signs |
|
|
Birds Chickens
Turkey
Geese & ducks
Sandhill/whooping cranes Parrots |
E. acervulina E. necatrix/maxima E. brunetti E. tenella E. mitis E. mivati E. praecox E. hagani
E. dispersa E. adenoeides E. meleagrimitis E. gallopavonis E. meleagridis E. innocua E. subrotunda E. truncata E. anseris/nocens E. reichenowi E. gruis E. psittaculae |
Duodenum/enteritis Mid-intestine/enteritis Ileum/enteritis Ceca/typhlitis NP SI/enteritis Duodenum/enteritis Watery intestinal contents, catarrhal inflammation Middle 1/3, +/- duodenum, cecum Cecum, ileum SI (anterior 2/3) Ileum, LI NP NP NP Kidney/anorexia, depression Intestine Disseminated
Intestine |
|
|
Cattle
Water buffalo calves |
E. bovis E. zuernii E. ellipsoidalis E. alabamensis E. auburnensis E. bukidnonensis E. kosti E. bareillyi |
Lower small intestine; HP Terminal meter of ileum; HP
SI; occas. LI Ileum
Abomasal glands
|
Common; diarrhea progresses to dysentery; hemorrhagic or fibrinohemorrhagic typhlocolitis; +/- mucosal ulceration; Heat-labile neurotoxin associated with development of nervous disorders in cattle with coccidiosis |
|
Sheep |
E. ahsata/christenseni E. bakuensis (ovina) E. crandallis E. ovinoidalis E. granulosa E. faurei E. parva E. intricata E. pallida E. caprovina E. punctata |
Lower SI Lower SI SI; villus atrophy Typhlocolitis |
Universal; young animals; E. bakuensis and E. ahsata can cause nodular polypoid structures NOT assoc. w/ clinical disease |
|
Goats |
E. christenseni E. arloingi E. hirci E. ninakohlyakimovea E. jolchijevi E. apsheronica E. alijevi E. kochrii E. weybridgenssis E. marsica E. caprina E. pallida E. caprovina E. punctata |
Lower SI Lower SI; occas. LI SI Typhlocolitis
SI
Typhlocolitis |
|
|
Equine |
E. leuckarti Klossiella equi |
SI (mainly foals) |
|
|
Swine |
E. debliecki Cystoisospora suis E. scabra E. spinosa |
SI (in 1-3 week old piglets) SI, distal 1/3 of villi Lower SI; most pathogenic |
C. suis - Porcine neonatal coccidiosis; fibrinonecrotic enteritis in distal SI; coccidiosis in older swine uncommon |
|
Canine |
Cystisospora canis C. ohioensis C. burrowsi C. neorivolta |
Distal SI; occas. LI SI, esp. ileum; LI SI SI; rarely cecum, colon |
|
|
Feline |
Cystoisospora felis C. rivolta |
SI; occas. LI SI and LI |
|
|
Mice |
E. falciformis E. vermiformis E. papillata E. ferrisi Klossiella muris |
Colon Intestine Intestine Intestine Kidney |
Typhlocolitis in pet and wild mice |
|
Rabbit |
E. stiedae E. intestinalis E. flavescens E. media E. magna E. piriformis E. irresidua E. perforans E. exigua E. coecicola (NP) |
Bile ducts Ileum and cecum Ileum and cecum |
Enteritis often accompanied by overgrowth of E. coli and presence of rotavirus |
|
Ferret |
E. furonis |
SI |
|
|
Przewalski’s Gazelle |
E. jiangi E. cagandzeeri |
|
|
|
Old world Camelids |
E. cameli E. dromedarii E. bactriani E. pellerdyi |
|
All have been associated with severe disease in OW camelids, especially the young. E. cameli is 3-4x larger than the others. |
|
HP = highly pathogenic; NP = non-pathogenic |
|
||
REFERENCES
- Abdul-Aziz T, Barnes HJ. Avian Histopathology Text and Atlas. Jacksonville, FL: American Association of Avian Pathologists; 2018: 154-158.
- Agnew D. Camelidae In: Terio K, McAloose D, Leger J, eds. Pathology of Wildlife and Zoo Animals, San Diego, CA: Elsevier 2018: 200-201.
- Barthold SW, Griffey SM, Percy DH. Pathology of Laboratory Rodents and Rabbits. 4th ed. Ames, IA: Blackwell Publishing; 2016: 82, 297.
- Boulianne M, et al. Avian Disease Manual. 8th Jacksonville, FL: AAAP; 2019:134-138.
- Cebra CK, Valentine BA, Schlipf JW, et al. Eimeria macusaniensis infection in 15 llamas and 34 alpacas. J Am Vet Med Assoc. 2007; 230(1): 94-100.
- Dubey JP. A review of coccidiosis in South American camelids. Parasitol Res. 2018;117(7): 1999-2013.
- Dubey JP, et al. Gametogony of Eimeria Macusaniensis. Parasitology 2018:145(12): 1540-1547.
- Hensel M, Bertram M, Rech R, Hamer G, Hamer S. Survey of gross and histopathologic findings in two wintering subpopulations of Sandhill cranes (Antigone canadensis). J Wildl Dis. 2018; 54(1): 156-160.
- Kwon YK, Jeon WJ, Kang MI, Kim JH, Olsen GH. Disseminated visceral coccidiosis in a wild white-naped crane (Grus vipio). J Wildl Dis. 2006; 42(3): 712-714.
- McDougald LR. Protozoal infections. In: Swayne DE, ed. Diseases of Poultry. 13th ed. Ames, IA: John Wiley & Sons, Inc.; 2013: 1148-1166.
- Schmidt RE, Reavill DR, Phalen DN. Pathology of Pet and Aviary Birds. 2nd Philadelphia, PA: John Wiley & Sons, Inc.; 2015: 82.
- Sledge DG, Bolin SR, Lim A, al. Outbreaks of severe enteric disease associated with Eimeria furonis infection in ferrets (Mustela putorius furo) of 3 densely populated groups. J Am Vet Med Assoc. 2011; 239(12): 1584-1588.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier; 2016: 227-235.
- Wang Y, Du S, Yang Y, et al. Intestinal parasites in the critically endangered Przewalski’s gazelle (Procapra przewalskii) in China, with the description of a new species of Eimeria (Apicomplexa: Eimeriidae). J Wildl Dis. 2016; 52(4): 945-948.
ENCLOSURE:
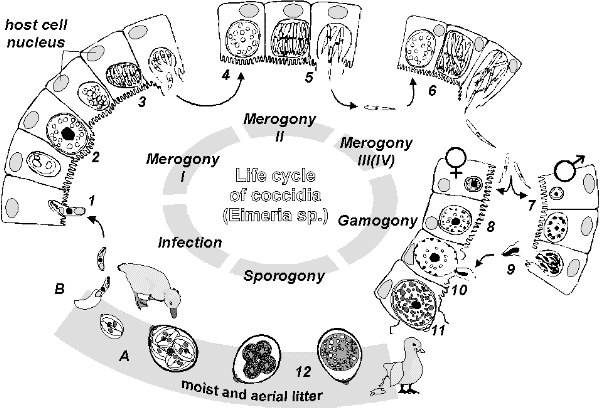
- Sporocyte (A) ingested à enzymatic degradation à free sporozoites invade enterocyte (1) à within parasitophorous vacuole develops into schizont (syn meront) (2/3) à merozoites form within meront à cell lysis and reinvasion (4/5) à 2nd and 3rd generation merozoites (6) à formation of gametes (7. male - microgamont, 8. female - macrogamont) à fertilization (9/10) à zygote à oocyst à discharged in feces (11) à sporulation in wet, warm soil (12) à sporocysts ingested (1)
Dipl. Biol. Andreas Weck-Heimann: Life cycle eimeria, http://www.saxonet.de/coccidia/coccid02.htm